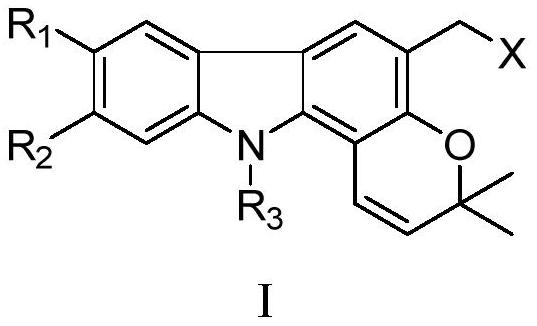
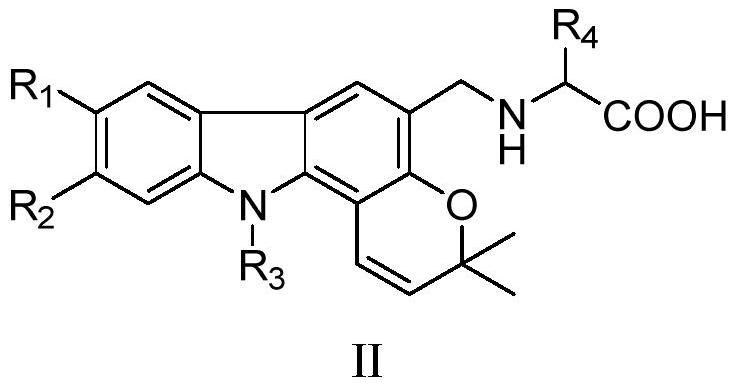
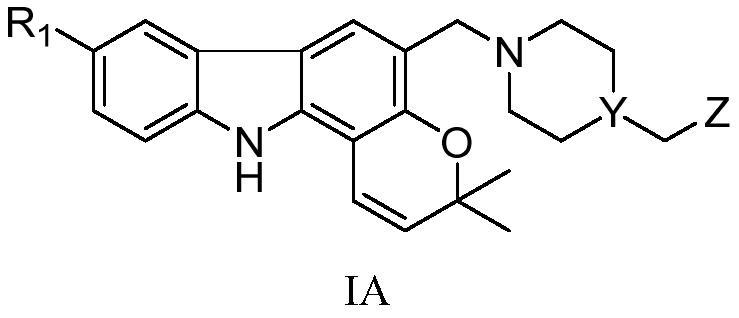
Pyranocarbazole alkaloid derivatives, and application thereof in treatment of nervous system diseases
A technology of pyranocarbazole and alkaloids, applied in the field of natural medicine and medicinal chemistry, can solve the problems of neuronal cell apoptosis, dysfunction, destruction of biomolecules, etc., to achieve increased solubility, avoid the risk of toxicity, The effect of improving bioavailability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0057] The preparation of intermediate 1 can refer to literature (European Journal of Medicinal Chemistry, 143, (2018) 438-448) and patent (a class of pyranocarbazole alkaloids and their preparation methods, pharmaceutical compositions and uses thereof, application number : 201611213048.6), the synthesis method is as follows:
[0058]
[0059] Wherein (a)~(e) is reaction condition: (a)Pd(OAc) 2 , BINAP, Cs 2 CO 3 , reflux, 3–16h; (b)Pd(OAc) 2 ,AcOH, 60℃, 24h; (c) (BAF, (HF, 0℃~room temperature, 30min; (d) 3-methyl crotonaldehyde, (i(OPr i ) 4 , anhydrous toluene, room temperature; (e) DDQ, mixed solvent methanol-tetrahydrofuran-water, room temperature.
[0060] When R 1 =C(CH 3 ) 3 , R 2 =H, it is intermediate 1a; when R 1 =OCH 3 , R 2 = H, intermediate 1b.
Embodiment 1
[0061] Example 1 Preparation of 8-(tert-butyl)-3,3-dimethyl-5-(4-methylpiperazine-1-methyl)-3,11-dihydropyran[3,2-a ] Carbazole(1)
[0062]
[0063] Intermediate 1a (200mg, 1eq) was dissolved in tetrahydrofuran solution, acetic acid (72mg, 2eq) and N-methylpiperazine (120mg, 2eq) were added, and stirred at room temperature for 0.5h. Then, sodium acetate borohydride (253mg, 2eq) was added thereto to continue the reaction for 5h, and the reaction was stopped. Spin the tetrahydrofuran solution to dryness, add 50ml of ethyl acetate, wash the ethyl acetate layer twice with saturated sodium bicarbonate solution (20ml×2), wash the ethyl acetate layer once with 20ml of saturated brine, dry over anhydrous sodium sulfate, filter, and the filtrate Spin dry to get crude product. Purified by silica gel column chromatography to obtain 198 mg of white powder with a yield of 78.9%.
[0064] 1 H NMR (500MHz, Methanol-d 4 )δ7.95(d, J=2.2Hz, 1H), 7.83(s, 1H), 7.38(dd, J=8.4, 2.2Hz, 1H), ...
Embodiment 2
[0067] Example 2 Preparation of 8-(tert-butyl)-3,3-dimethyl-5-((4-(3-methylbenzyl)piperazin-1-yl)methyl)-3,11-di Hydropyrano[3,2-a]carbazole(2)
[0068] Intermediate 1a (200mg, 1eq), acetic acid (72mg, 2eq), sodium acetate borohydride (253mg, 2eq), 1-(3-methylbenzyl)piperazine (228mg, 2eq), refer to the synthesis of Example 1 Method, 200 mg of white powder was obtained with a yield of 65.7%.
[0069] 1 HNMR (400MHz, Acetone-d 6 )δ10.33(s,1H),8.08(s,1H),8.06(d,J=1.6Hz,1H),7.40(dd,J=1.6,8.8Hz,1H),7.35(d,J=8.8 Hz, 1H), 7.19-7.04(m, 4H), 6.94(d, J=9.8Hz, 1H), 5.78(d, J=9.8Hz, 1H), 3.96(s, 2H), 3.46(s, 2H ),2.85(s,4H),2.61(s,4H),2.28(s,3H),1.47(s,6H),1.40(s,9H).
[0070] 13 C NMR (100MHz, Acetone-d 6 )δ150.8, 143.0, 139.5, 139.4, 138.6, 138.0, 130.7, 130.2, 129.1, 128.7, 127.1, 124.4, 123.7, 123.4, 118.7, 118.5, 116.7, 111.2, 111.61, 105.3, 6.1, 23.1, 77 ,35.3,32.5,28.0,21.5.
[0071] HRESIMS m / z=508.33182[M+H] + (calcd for C 34 h 42 ON 3 ,508.33224).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com