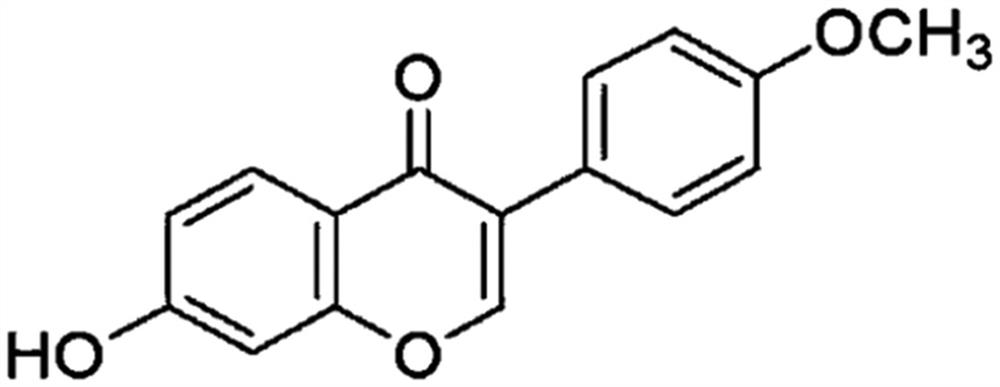
Application of formononetin in preparation of medicines for treating or/and preventing depression
A technology of formononetin and depression, which is applied in the field of biomedicine, can solve the problems of significant side effects, central nervous system inflammation and depression, low side effects, and lack of effective treatment, so as to achieve small side effects and adverse reactions Small, low-cost effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] The embodiment of the present application provides different modeling treatments for rats, including:
[0049] Thirty healthy male SD rats were selected, aged about 6 weeks, and weighing between (200±20) g. In a clean animal room, room temperature (21±2)°C, relative humidity 30%-40%, light and dark for 12 hours, light time from 7:00 am to 19:00 pm, and dark time from 19:00 pm to 7:00 am the next day Time. Rats were fed adaptively for one week, and were randomly divided into experimental groups after weighing.
[0050] The animal model of depression-like behavior was prepared by intraperitoneal injection of LPS. The LPS was dissolved in sterile water and the injection volume was 1 mg / mL. It was fed at 9 am on the first day of model establishment for two consecutive days. The dose of formononetin in the lipopolysaccharide LPS+formononetin administration group was 30 mg / kg·d, and the intragastric volume was 1 mL / 100g body weight.
[0051]The experimental rats were rando...
Embodiment 2
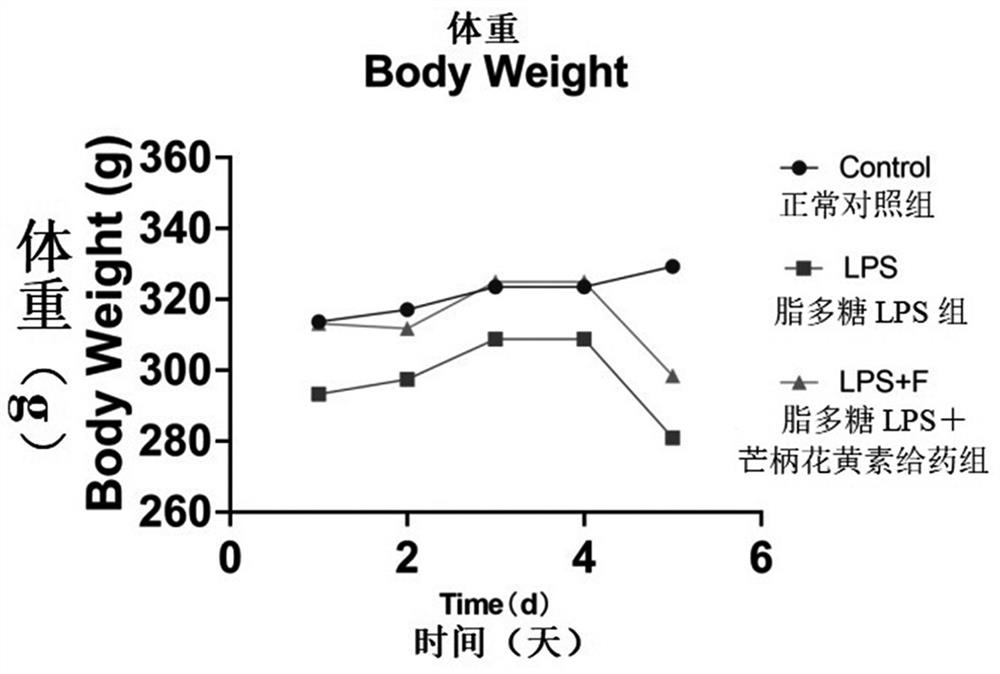
[0053] This embodiment provides the general state and body weight changes of the Control group, LPS group and LPS+F group of embodiment 1, specifically including:
[0054] The mental state, fur color, and autonomous activity of the rats were observed daily. From the day before modeling, the rats were weighed and recorded every day to observe and compare the changes in the weights of the rats in each group.
[0055] One day before modeling, the rats in each group were in good mental state, with black and soft fur, flexible movements, spherical feces and moderate dryness and wetness. On the 5th day after modeling, the rats in the LPS group had a poor mental state, messy hair, yellow color, and decreased activity. The mental state of the rats in the LPS+F group was acceptable, the hair was more tidy, and the movements were sensitive and lively. Body weight analysis of rats as figure 1 Shown: On the 5th day of modeling, compared with the Control group, the body weight of the ra...
Embodiment 3
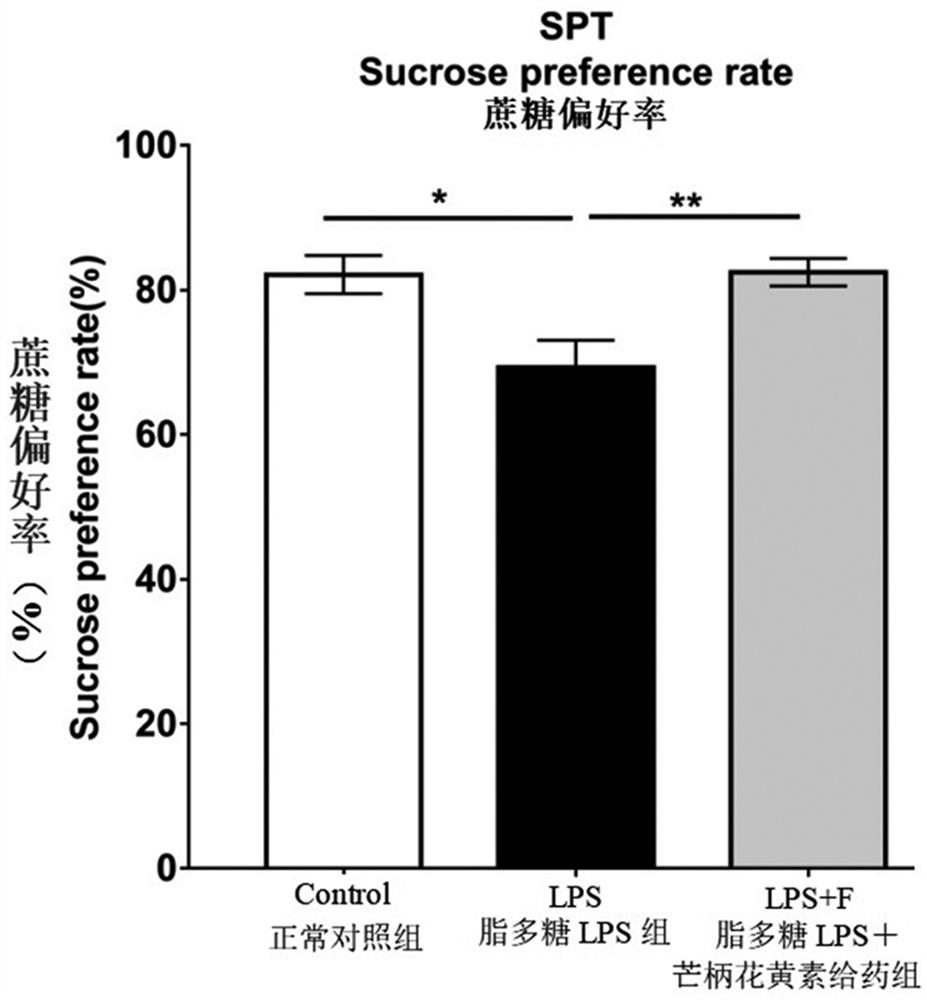
[0057] The present embodiment provides the sugar water preference test of the Control group, the LPS group and the LPS+F group of embodiment 1, specifically including:
[0058] Observe the depression-like behavior of the Control group, LPS group and LPS+F group experimental animals of Example 1. Start the experimental process in a quiet environment. The experiment started training two days in advance. On the first day, rats were given a bottle of sugar water (1% sucrose) and a bottle of pure water, and on the second day, rats were given two bottles of pure water. After fasting for 24 hours on the third day, the rats were placed in a cage with a bottle of sugar water (1% sucrose) and a bottle of pure water, and the amount of sugar water and pure water that the rats drank within 4 hours was calculated and calculated. Statistics of their sugar water preference rate. Sugar water preference rate = the amount of drinking sugar water / (the amount of drinking sugar water + the amou...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com