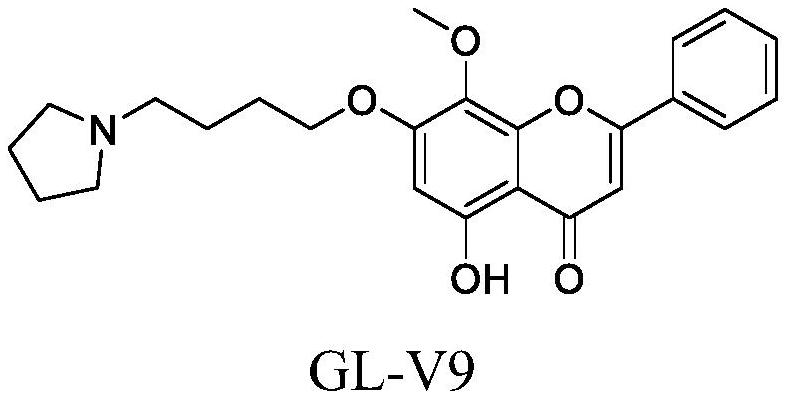
Application of GL-V9 in preparation of anti-glioma drugs
A technology for GL-V9 and glioma, applied in the field of anti-tumor drugs, can solve problems such as easy recurrence, poor prognosis, and fast growth, and achieve the effect of promoting early and late apoptosis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
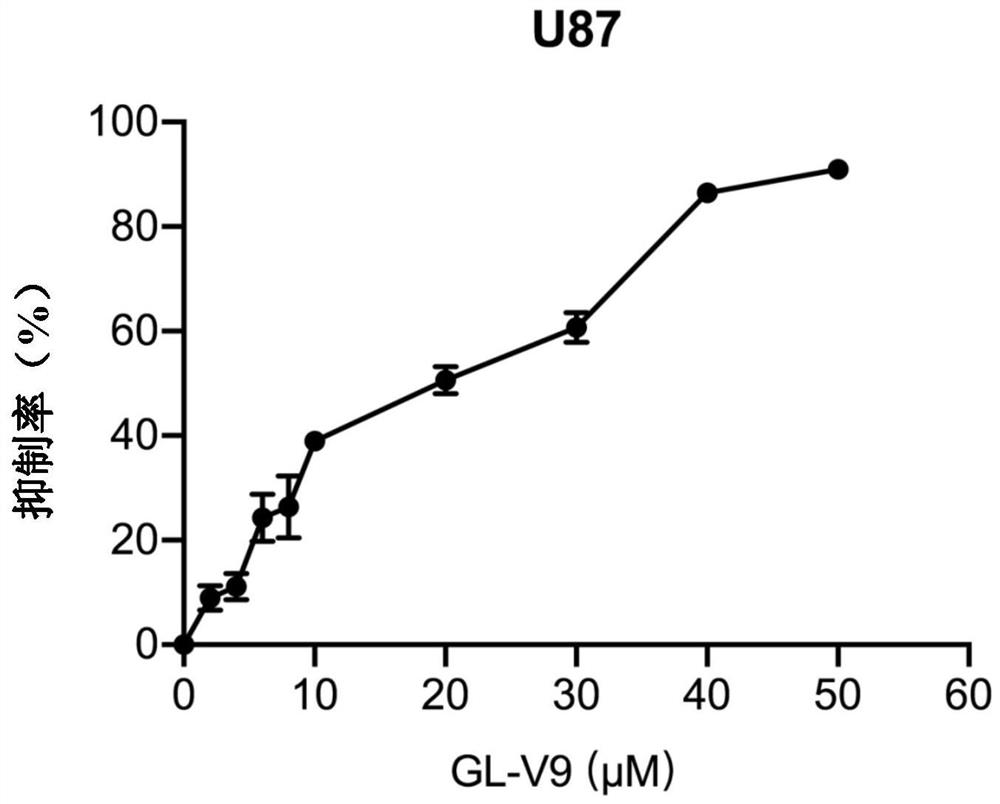
[0043] Embodiment 1 MTT experiment
[0044] MTT solution can be reduced to blue-purple crystalline formazan by mitochondrial dehydrogenase in living cells, DMSO can dissolve formazan, and the color of the formed solution is proportional to the cell viability, so the cell viability can be detected. Cells were cultured in a 96-well ELISA plate according to the appropriate cell density, and the volume of cells in each well was 100 μl, and 100 μl of GL-V9 at a specified concentration was added to it at the same time; the cells given the drug were continued to be cultured in the incubator for 24 hours Afterwards, 20 μl MTT solution was added to each well; the supernatant was removed after continued incubation for 4 h, and 100 μl DMSO was added to each well, placed in a micro-oscillator to vibrate, and after the crystals were completely dissolved, the absorbance was detected with a wavelength of 570 nm. After sorting out the detected absorbance values, calculate the growth inhibitio...
Embodiment 2
[0047] Example 2 Annexin V / PI apoptosis double staining experiment
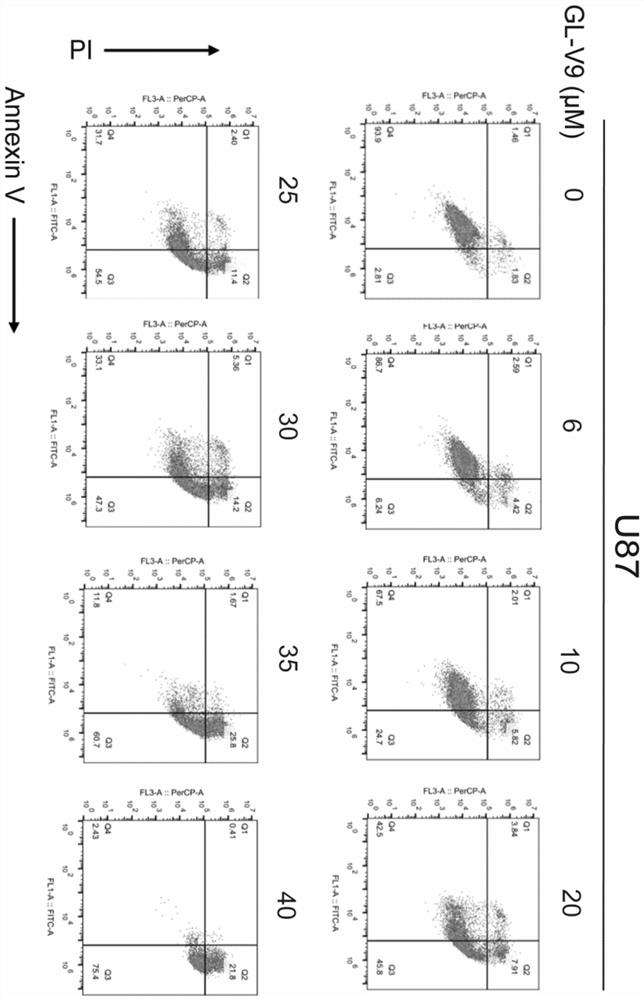
[0048] After the specified time of drug action, centrifuge at 2000rpm for 5min to collect the cell suspension, discard the medium, wash the cells twice with pre-cooled PBS solution, add 400μL 1×Binding Buffer to suspend the cells, and the cell density is about 1×10 6 individual / mL. Add 5 μL of Annexin V to the cell suspension of each group, mix gently and incubate at 2-8°C in the dark for 15 minutes, then add 5 μL of PI, mix gently and incubate at 2-8°C in the dark for 5 minutes. Within 1 h, it was detected by flow cytometry.
[0049] The effect of natural product derivative GL-V9 on the apoptosis of glioma cell line U87 at 24h time point was determined by flow cytometry. The results of Annexin V / PI double staining experiment showed that ( figure 2 ), GL-V9 can significantly promote early and late apoptosis of U87 cells. These results preliminarily confirmed that GL-V9 plays an anti-glioma role by induci...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com