Pyruvate dehydrogenase mutant and method for producing L-amino acid by using mutant
A technology of pyruvate dehydrogenase and mutants, applied in the direction of microorganism-based methods, biochemical equipment and methods, botany equipment and methods, etc., can solve problems such as no new mutation sites and achieve improved conversion rate , the effect of increasing production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
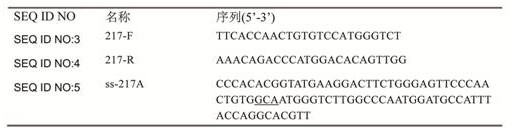
[0081] Example 1. Editing plasmid pCas9gRNA- aceE 217 builds
[0082] The present invention has carried out in-depth analysis on the AceE enzyme, and it is predicted that position 217 may be a site that affects its activity, so the site is mutated for follow-up research. Subsequently, according to the Goldengate cloning method reported in the literature (WANG, Yu, et al. Expanding targeting scope, editing window, and base transition capability of base editing in Corynebacterium glutamicum . Biotechnology and bioengineering, 2019, 116: 3016-3029) to construct targeted aceE The pCas9gRNA plasmid of the 217th amino acid residue codon of the gene, the target DNA binding region of the sgRNA is CCAACTGTGTCCATGGGTCT. The specific method is as follows: 217-F / 217-R is denatured and annealed to obtain a DNA double-stranded product with sticky ends, and then combined with pCas9gRNA- ccdB The plasmid (reference CN112111469B) was cloned with Goldengate (NEB® Golden Gate Assembly Kit, ...
Embodiment 2
[0087] Example 2. Construction of Corynebacterium glutamicum aceE The mutant of the 217th amino acid codon mutation in the gene
[0088] Since wild-type Corynebacterium glutamicum cannot produce lysine, the introduction of lysC , pyc and hom Lysine can be produced after the point mutation. In this embodiment, ATCC13032 is used as the starting strain, and a lysine-producing bacterial strain is constructed at first, that is, the aspartokinase gene in Corynebacterium glutamicum ATCC13032 strain lysC Introduced the T311I point mutation (relieves feedback inhibition of the enzyme) in the pyruvate carboxykinase gene pyc Introduction of the P458S point mutation (relieves feedback inhibition of the enzyme) in the homoserine dehydrogenase gene hom Introduce the V59A point mutation (weaken the activity of the enzyme) to obtain the lysine high-yielding strain AHP-3.
[0089] Subsequently, using the CRISPR / Cas9 genome editing system based on single-strand recombination (refer ...
Embodiment 3
[0092] Example 3. Corynebacterium glutamicum aceE The effect of gene 217 mutation on L-lysine synthesis
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com