Cloperadine hydrochloride tablet and preparation method thereof
The cloperidine hydrochloride tablet and the cloperidine hydrochloride technology are applied in the directions of pharmaceutical formulations, medical preparations with inactive ingredients, medical preparations containing active ingredients, etc., and can solve the problems of complex preparation process, low production efficiency and the like, Achieve the effect of saving process steps, improving production efficiency, and consistent efficacy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
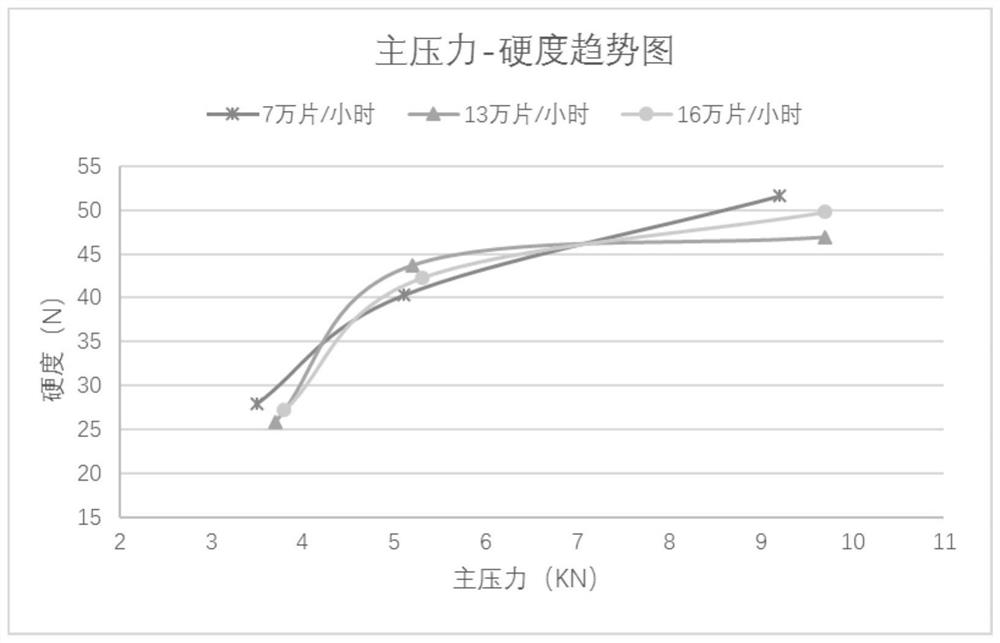
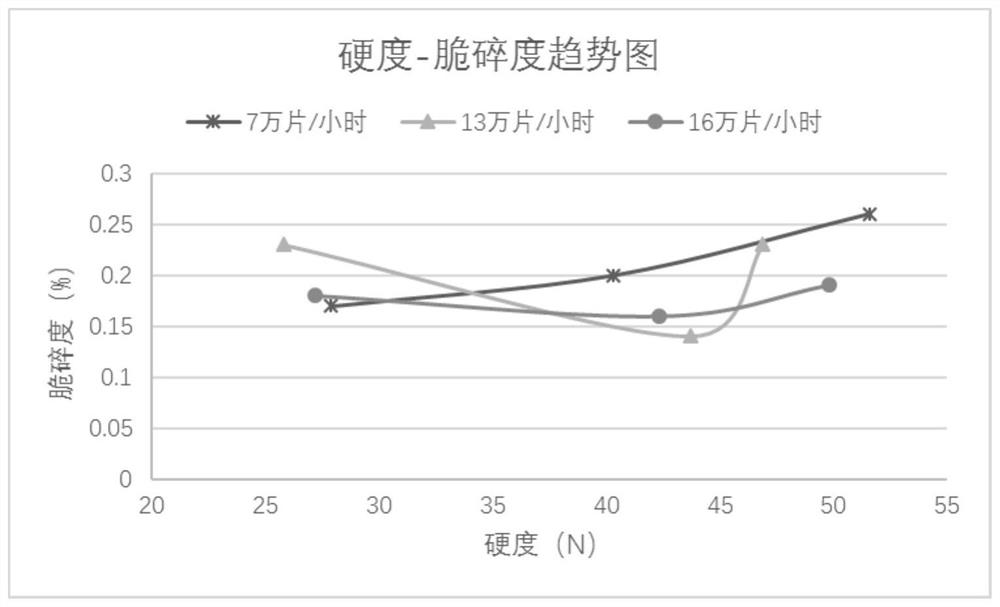
[0061] According to the above steps, a small test is carried out first, and after the data of the small test is qualified, two amplification operations are carried out.
[0062] 1. Preprocessing stage
[0063] The twice-amplified batch ingredients list is shown in Table 4:
[0064] Table 4 Enlarged Batch Ingredients List
[0065]
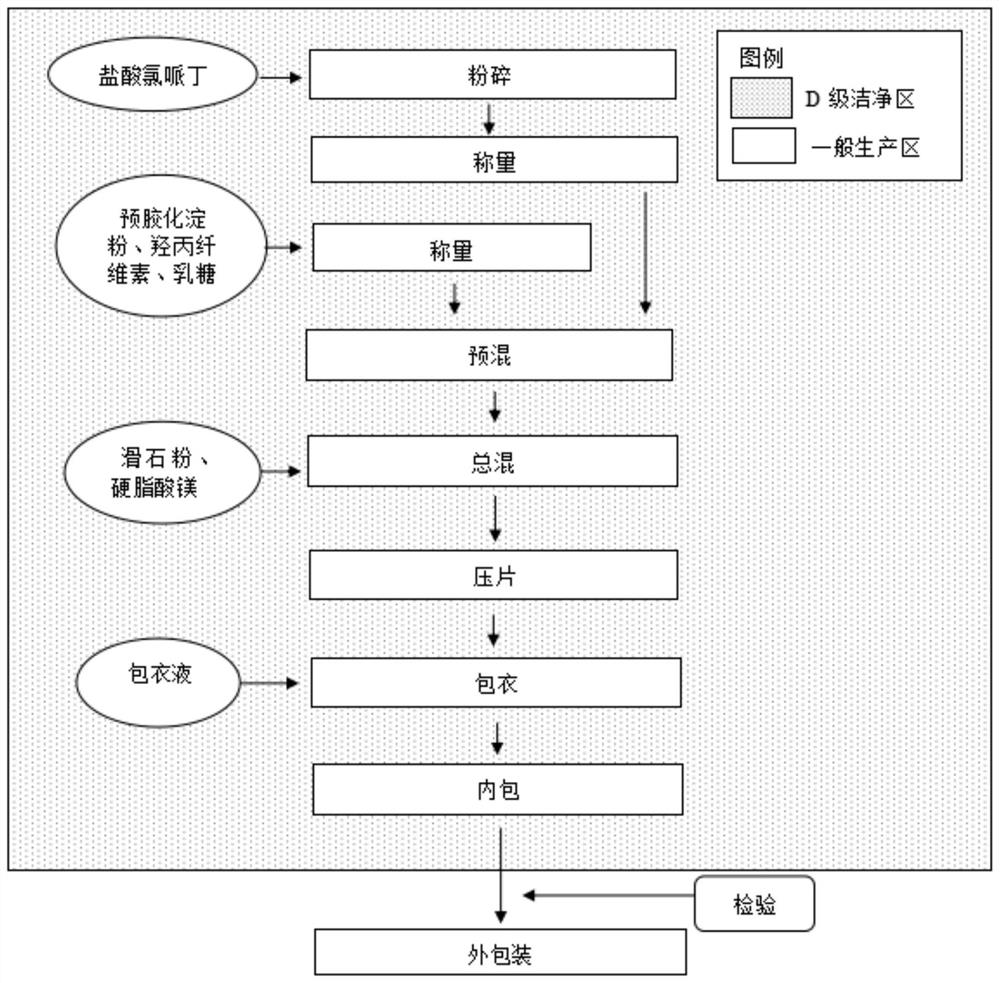
[0066] As shown in Table 5, cloperidine hydrochloride is pulverized by a vacuum pulverizing unit, which meets the production requirements:
[0067] Table 5 Material balance and yield calculation of raw material drug pretreatment process
[0068] project (1) batch (2) batch acceptable standard Material Balance(%) 97.7 97.6 N / A Yield (%) 97.3 97.5 N / A
[0069] 2. Premixing stage
[0070] Two batches of amplification, the premixing parameters are shown in Table 6:
[0071] Table 6 hydroxypropyl cellulose, pregelatinized starch pretreatment process material balance and yield calculation
[0072] pro...
PUM
| Property | Measurement | Unit |
|---|---|---|
| hardness | aaaaa | aaaaa |
| friability | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com