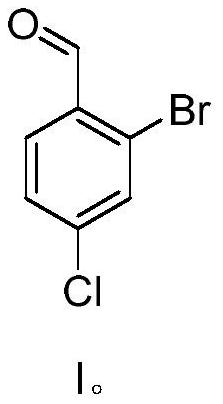
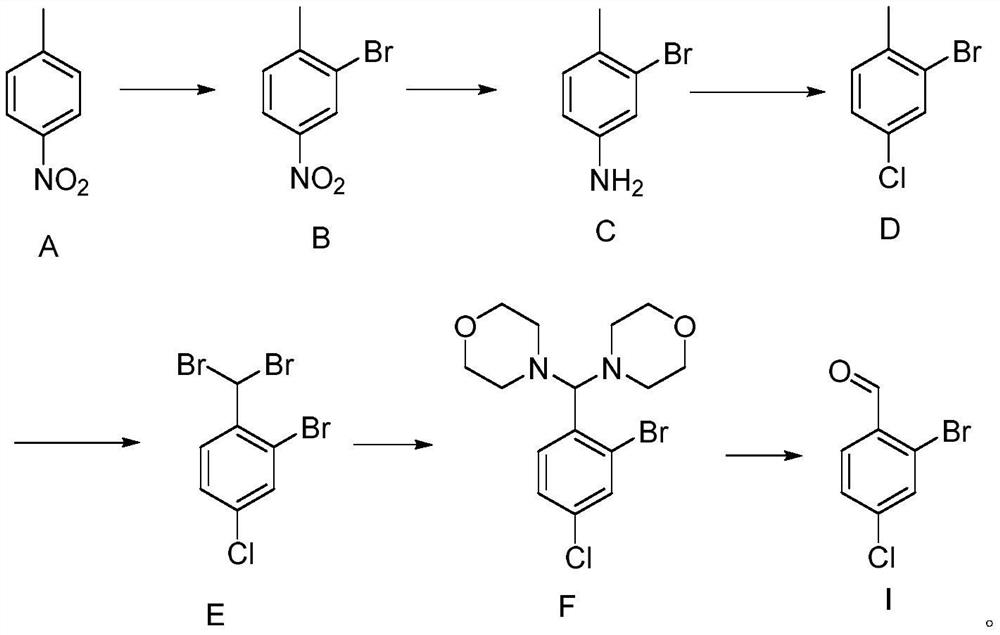
Preparation method of 2-bromo-4-chlorobenzaldehyde
A technology of toluene and formic acid, which is applied in the field of preparation of 2-bromo-4 chlorobenzaldehyde, can solve the problems of excessive three wastes, non-environmental protection, and complicated post-treatment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0071] The preparation of embodiment 1 compound B
[0072]
[0073] Add 40L of 50% sulfuric acid to the 100L reaction kettle, stir, and slowly add compound A (5kg) that has been melted in advance. Reaction at ℃ for 4-12 hours, HPLC monitoring of the reaction end point, after the reaction, cool down to room temperature, add 40L of water and stir for 2 hours, filter with suction, wash the filter cake with a small amount of water until the filtrate is neutral, drain it, and dry it under normal pressure at 50-55°C to obtain compound B ( 7.7kg), yield 97.8%, purity 98%.
Embodiment 2
[0074] The preparation of embodiment 2 compound C
[0075]
[0076] Add 75L of ethanol and compound B (7.5kg) to a 100L reaction kettle at room temperature, stir and heat up to 60-80°C, after dissolving, add Raney Ni (0.75kg), control the temperature at 60-80°C, and add 80% hydration solution dropwise Hydrazine (10kg), dropwise reacted for 4-12h, TLC controlled the reaction end point, after the reaction, cooled to normal temperature, filtered to remove Raney Ni (be careful not to drain), the filtrate was concentrated and evaporated to remove the solvent to obtain compound C (6.3kg), Yield 97.5%, purity 97%.
Embodiment 3
[0077] The preparation of embodiment 3 compound D
[0078]
[0079] Add 25L of 15% hydrochloric acid to a 50L reactor, below 20°C, slowly add compound C (5kg), control the temperature below 10°C, add dropwise sodium nitrite aqueous solution (2kg dissolved in 6L of water) to complete the reaction for 1-2h, HPLC Monitor the end point of the reaction, and keep it ready for use after the reaction is completed.
[0080] Add 10L of 15% hydrochloric acid to the 100L reactor, then add CuCl (4kg), drop the reaction liquid in the above 50L reactor into the 100L reactor, control the temperature at 0-10°C, finish the reaction for 2-5 hours, and monitor the reaction by HPLC End point, after the reaction is over, add DCM (15L) to separate layers, extract the aqueous layer with DCM (5L), combine the organic layers, add 10L water to wash twice, evaporate the organic layer to remove DCM, and distill the crude product under reduced pressure to obtain compound D (5kg) , yield 90.5%, purity 9...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com