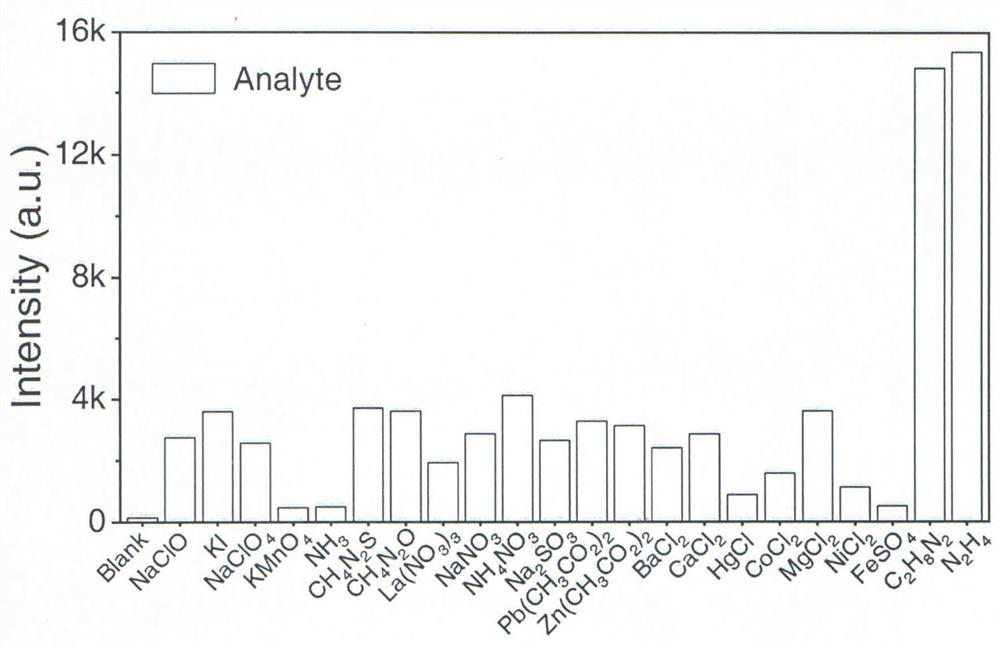
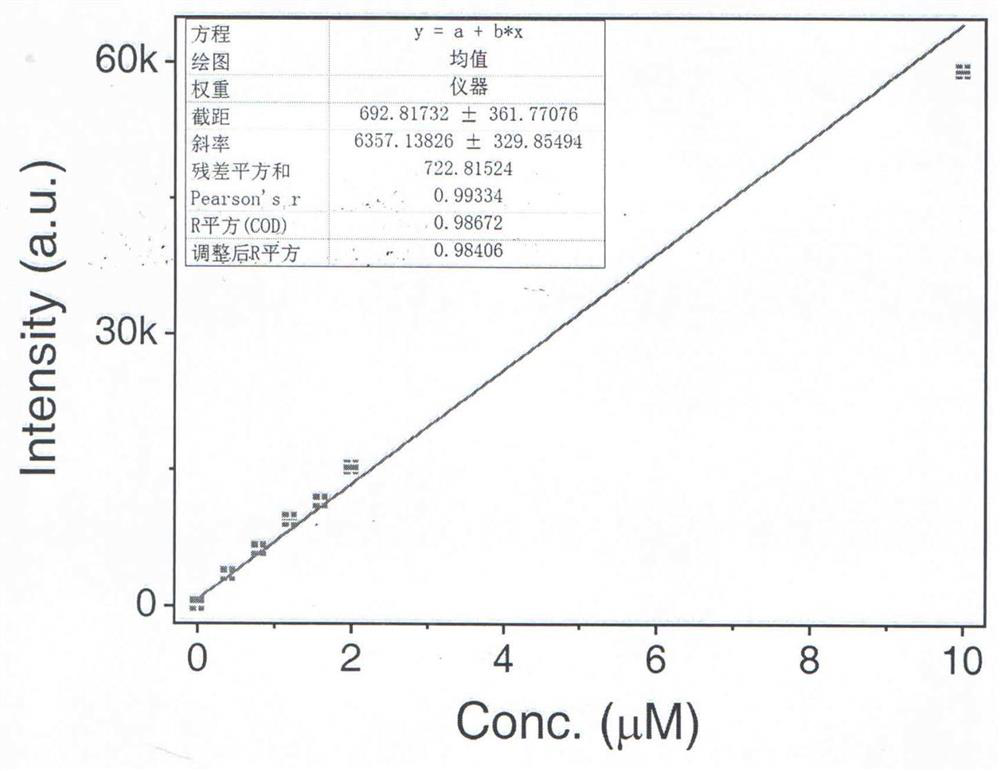
Hydrazine visualization fluorescent probe molecule and preparation method thereof
A fluorescent probe and molecular technology, applied in chemical instruments and methods, fluorescence/phosphorescence, material analysis through optical means, etc., to achieve stable results, simple production, and good specificity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
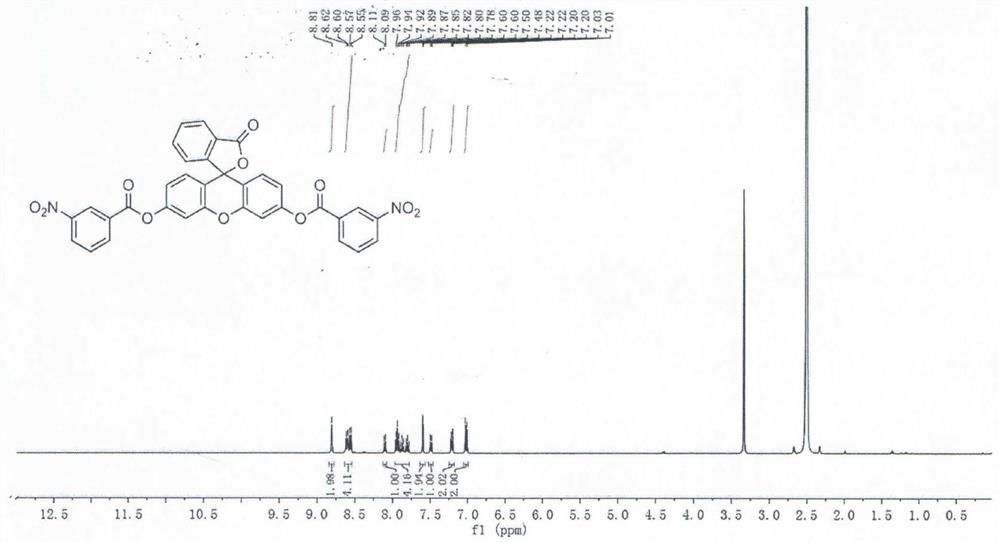
[0032] Synthesis of fluorescent probe molecule 3-oxo-3H-spiro[isobenzofuran-1,9'-oxanthene]-3',6'-diacylbis(3-nitrobenzoate):
[0033] a, 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride 2mmol, 0.384g, 4-dimethylaminopyridine 2mmol, 0.244g and 3-nitrobenzoic acid 6mmol, Mix 1.002g into a 200mL round-bottomed flask, then add 50mL of dichloromethane, stir at room temperature for 10min, and form a clear and transparent solution after completely dissolving, then add 2mmol of fluorescein, 0.664g, and stir at room temperature for 24h to stop the reaction;
[0034]b. After the reaction is cooled to room temperature, add 100 mL of dichloromethane, fully dissolve, filter to remove impurities, and then use vacuum distillation to obtain the crude product;
[0035] c. Purify the crude product obtained in step b through a silica gel column, wash with dichloromethane:ethyl acetate eluent with a volume ratio of 15:1, and then dry the eluted product to obtain a white solid for dete...
Embodiment 2
[0039] Synthesis of fluorescent probe molecule 3-oxo-3H-spiro[isobenzofuran-1,9'-oxanthene]-3',6'-diacylbis(3-cyanobenzoate):
[0040] a, 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride 3mmol, 0.576g, 4-dimethylaminopyridine 3mmol, 0.366g and 3-cyanobenzoic acid 9mmol, 1. Mix 323g into a 300mL round-bottomed flask, then add 75mL of dichloromethane, stir at room temperature for 10min, after completely dissolving, it becomes a clear and transparent solution, then add 3mmol of fluorescein, 1.0g, stir at room temperature for 24h, stop the reaction;
[0041] b. After the reaction is cooled to room temperature, 150 mL of dichloromethane is added by volume ratio, fully dissolved, filtered to remove impurities, and then the crude product is obtained by distillation under reduced pressure;
[0042] c. Purify the crude product obtained in step b through a silica gel column, wash with dichloromethane:ethyl acetate eluent with a volume ratio of 20:1, and then dry the eluted pr...
Embodiment 3
[0045] Synthesis of fluorescent probe molecule 3-oxo-3H-spiro[isobenzofuran-1,9'-xanthene]-3',6'-diacylbis(3-fluorobenzoate):
[0046] a, 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride 2mmol, 0.384g, 4-dimethylaminopyridine 2mmol, 0.244g and 3-fluorobenzoic acid 6mmol, 1.002 g to mix, add to a 200mL round-bottomed flask, then add 50mL of dichloromethane, stir at room temperature for 10min, after completely dissolving, it becomes a clear and transparent solution, then add 2mmol of fluorescein, 0.664g, stir at room temperature for 24h, stop the reaction;
[0047] b. After the reaction is cooled to room temperature, add 100 methylene chloride by volume, fully dissolve, filter to remove impurities, and then use vacuum distillation to obtain the crude product;
[0048] c. Purify the crude product obtained in step b through a silica gel column, wash with dichloromethane:ethyl acetate eluent with a volume ratio of 15:1, and then dry the eluted product to obtain a white s...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com