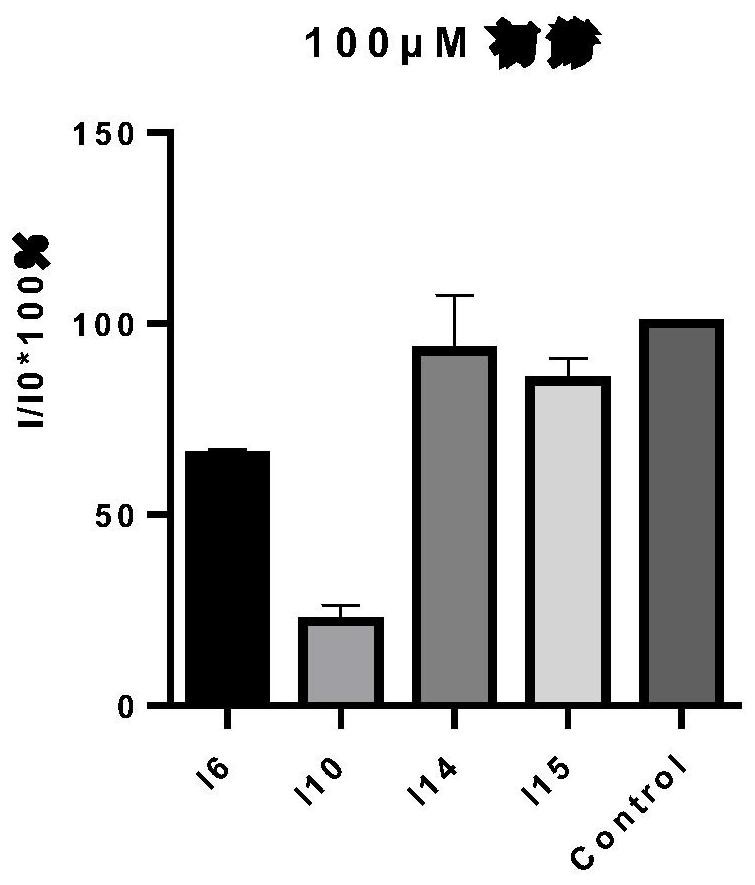
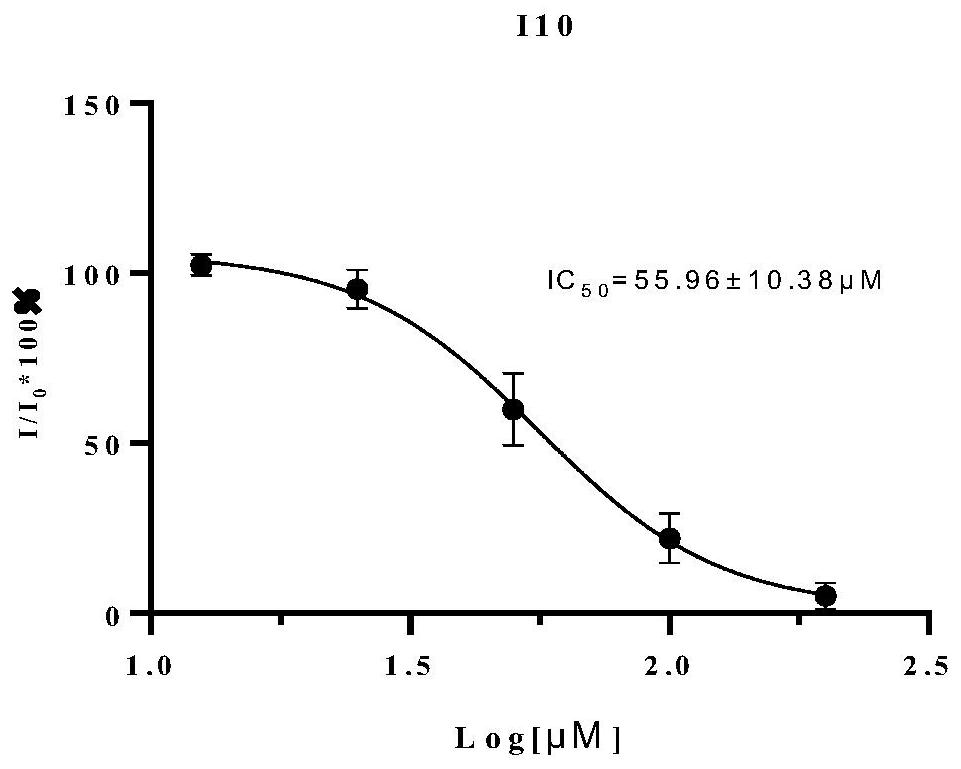
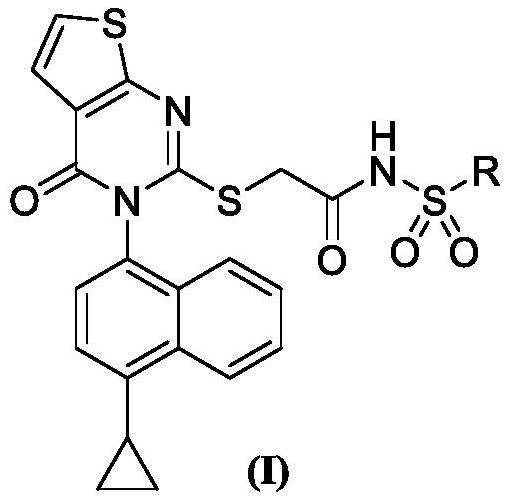
A kind of thienopyrimidinone acylsulfonamide derivatives and preparation method and application thereof
A technology for pyrimidinone acyl sulfonamides and derivatives, which is applied in the field of thienopyrimidinone acyl sulfonamide derivatives and their preparation, and can solve the problems of poor curative effect, toxic and side effects, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Example 1. Intermediate 2-((3-(4-Cyclopropylnaphthalen-1-yl)-4-oxo-3,4-dihydrothieno[2,3-d]pyrimidin-2-yl ) Preparation of thio) acetic acid (I-5)
[0035] Preparation of intermediate 4-cyclopropyl-1-naphthylamine (I-1):
[0036] 4-Bromo-1-naphthylamine (3.0 g, 13.51 mmol), cyclopropylboronic acid (1.5 g, 17.44 mmol), K 3 PO 4 (10.2g, 48.05mmol), tetrakis (triphenylphosphine) palladium (1.5g, 1.3mmol) were successively added to a 250mL double-neck flask, 50mL of toluene and 4mL of distilled water were added as solvents, mixed well, N 2 Under the protection, the reaction was heated and refluxed at 100 °C for 12 h; after monitoring the completion of the reaction by TLC, the reaction solution was cooled to room temperature, filtered through celite, the filtrate was evaporated to dryness, the residue was dissolved in ethyl acetate, and washed with saturated NaCl solution (50 mL × 3 times), the organic phases were combined, dried over anhydrous sodium sulfate, and filtere...
Embodiment 2
[0045] Example 2. Preparation of Compound I1
[0046] Intermediate I-5 (0.2 g, 0.49 mmol) was dissolved in 5 mL of dry dichloromethane, stirred in an ice bath for 10 min, added DMAP (0.09 g, 0.74 mmol), continued to stir in an ice bath for 10 min, and then added EDCI (0.14 g) , 0.74 mmol), and finally, after stirring in an ice bath for 30 min, benzenesulfonamide (0.085 g, 0.54 mmol) was added, slowly raised to room temperature, and stirred for 15 h; monitored by TLC, after the reaction was completed, the solvent was evaporated under reduced pressure, and acetic acid was added to the residue. Ethyl ester 20mL, successively with saturated NaHCO 3 , 1 mol / L dilute hydrochloric acid, washed with saturated NaCl solution (20 mL × 2 times), combined the organic phases, dried over anhydrous sodium sulfate, filtered, the filtrate was concentrated, and separated by column chromatography (methanol: dichloromethane: glacial acetic acid = 1: 30:1.5%). White solid, yield 48.5%, melting po...
Embodiment 3
[0048] Example 3. Preparation of compound I2
[0049] The operation is the same as that in Example 2, except that the sulfonamide used is 4-nitrobenzenesulfonamide, white solid, yield 41.4%, melting point: 176-180°C.
[0050] Compound I2 spectral data: 1 H NMR (400MHz, DMSO-d 6 )δ12.70(s, 1H), 8.53(d, J=8.5Hz, 1H), 8.18(d, J=8.7Hz, 2H), 7.90(d, J=8.5Hz, 2H), 7.66(t, J=7.7Hz, 1H), 7.53 (dd, J=14.8, 6.8Hz, 3H), 7.41 (dd, J=8.0, 5.3Hz, 2H), 7.35 (d, J=5.8Hz, 1H), 3.75– 3.65 (m, 2H), 2.58–2.54 (m, 1H), 1.15 (dd, J=8.7, 4.2Hz, 2H), 0.93–0.81 (m, 2H). 13 C NMR (100MHz, DMSO-d 6 )δ171.31,164.05,162.79,160.38,157.99,151.90,148.56,142.31,134.14,131.02,129.71,128.57,128.19,127.88,127.09,125.43,123.54,123.39,122.91,122.76,122.44,120.64,36.26,13.43,7.72 ,7.39.ESI-MS:m / z 591.06[M-H] - ,C 27 H 20 N 4 O 6 S 3 [592.05].
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com