Novel application of triterpenoid acid compound as A beta fiber formation inhibitor drug
A technology of compounds and inhibitors, which is applied in the field of triterpenic acid compounds as Aβ fiber formation inhibitor drugs, to achieve the effect of improving behavioral ability, inhibiting the formation of Aβ fibers, and reducing Aβ content
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0083] Validation and screening of Aβ fibrosis inhibitors in Scutellaria baicalensis
[0084] Yu L, Wu A G, Wong K W, et al. The New Application of UHPLC-DAD-TOF / MS in Identification of Inhibitors on β-Amyloid Fibrillation From Scutellaria baicalensis[J].other,2019,10. In this journal, potential inhibitors of Aβ fibrosis in Scutellaria baicalensis were screened by pre-incubating Aβ(1-42) with Scutellaria baicalensis extract and then analyzed by UHPLC-DAD-Q / TOF-MS / MS. Compared with Scutellaria baicalensis extract alone, xanthosides and xanthosides were found to be significantly lower in the Aβ-containing culture medium, indicating that xanthosides and xanthosides are potent inhibitors of Aβ fibrosis in Scutellaria baicalensis.
[0085] Embodiment 1 uses the high-throughput screening method provided by the present invention to verify
[0086] Preparation of Scutellaria baicalensis extract
[0087] Scutellaria baicalensis was weighed and pulverized, then distilled and extra...
Embodiment 2
[0103] Preparation of Kaixinsan Extract
[0104] Weigh and crush Kaixin San (the Kaixin San contains ginseng, Polygala, Shichangpu, and Poria cocos), then distill and extract with 10 times the volume of water for 1.5 hours, repeat the extraction 4 times, combine the extracts, filter, concentrate, and then The concentrated extract was dissolved into a 400 mg / mL solution with DMSO (dimethyl sulfoxide) to obtain the Chinese herbal medicine extract to be tested.
[0105] Preparation of biotinylated Aβ(1-42) peptide
[0106] 5 mg of Aβ(1-42) peptide was dissolved in hexafluoroisopropanol (HFIP, Sigma) solution, then the HFIP solution was dispensed into new 1.5 mL test tubes and dried under nitrogen flow, then, in the test tubes Add 10 μL of DMSO (dimethyl sulfoxide) and an appropriate volume of PBS (comprising 15% DMSO and 0.02% Tween 20) to obtain a working concentration of 100 mg / mL of Aβ(1-42).
[0107] Biotin (EZ-LinkNHS-LC-LC-Biotin (Thermo Scientific, USA)) with a molar...
Embodiment 3
[0124] Separation and purification of PPAC, DTA and TA from Poria cocos
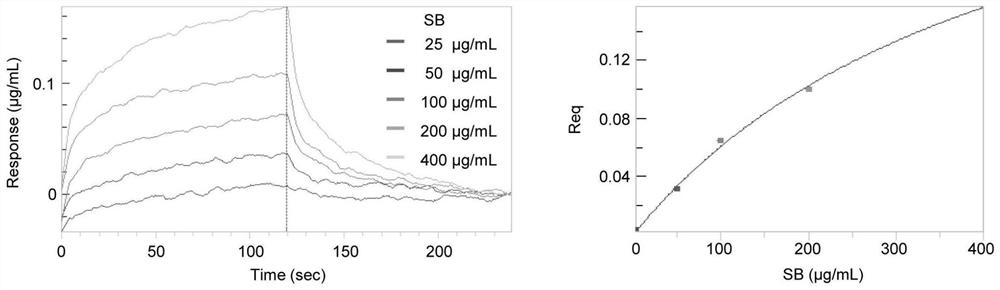
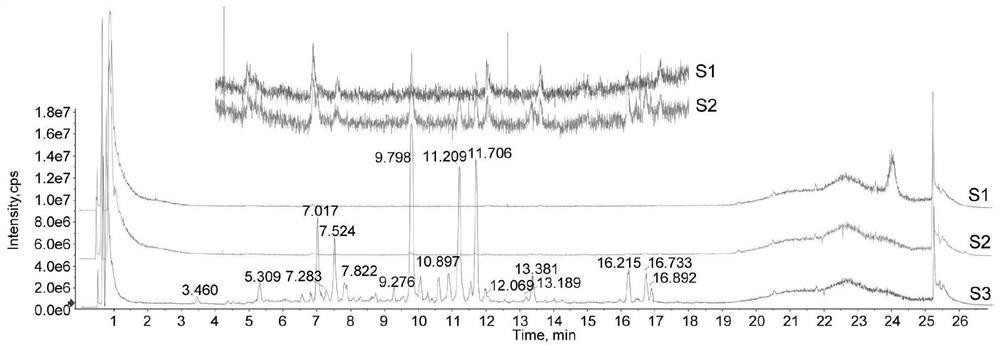
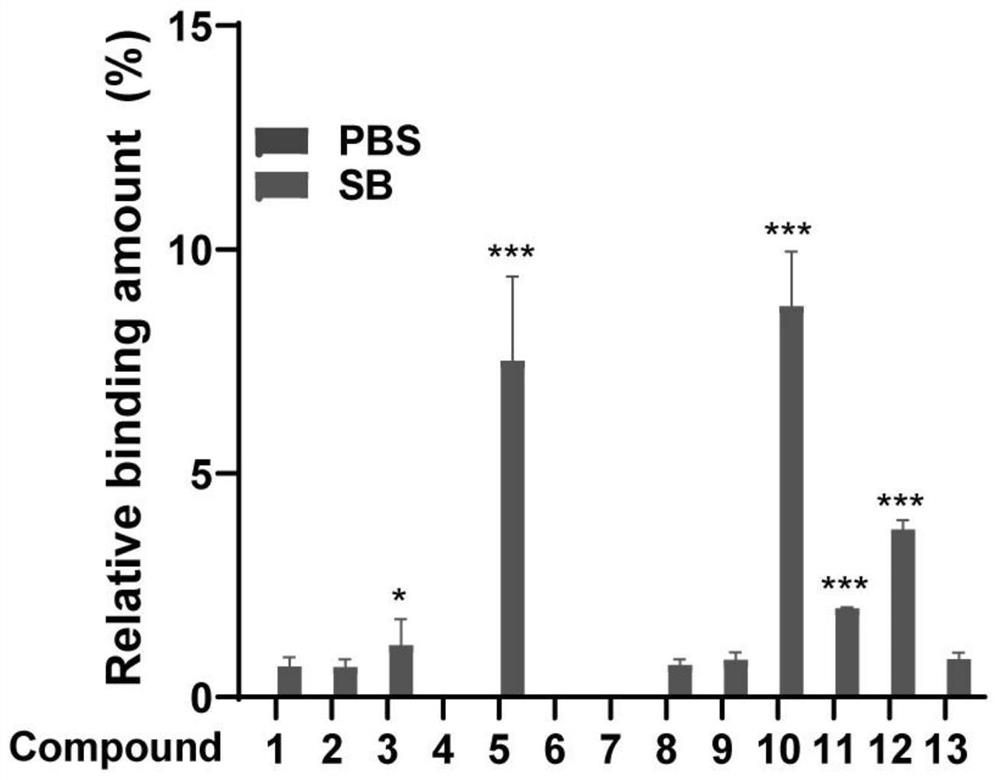
[0125] Firstly, take Poria cocos powder, soak it with ethyl acetate reagent, and then use a rotary evaporator to rotate and collect, and repeat 3 times to obtain Poria cocos extract. The ethyl acetate extract of Poria cocos was roughly separated using a silica gel chromatography column, and 100ml fractions were collected and divided into one group, and then the target compound was detected by mass spectrometry, and finally purified by ultra-fast high-performance liquid phase. Finally, three compounds were successfully isolated, including pyripolic acid C, dehydroturmoic acid, and turmolic acid.
[0126] Figure 9 The representative total ion chromatograms in show the UHPLC-DAD-Q / TOF-MS / MS analysis of PPAC, DTA, TA and Poria cocos extract. Among them, S1: Poria cocos extract; S2: PPAC; S3: DTA; S4: TA. From Figure 10 It shows that the measured accurate masses of PPAC, DTA and TA are [MH]-481.3388, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com