M-MLV reverse transcriptase mutant and application thereof
A technology of reverse transcriptase and mutants, which is applied in the field of M-MLV reverse transcriptase mutants, can solve the problems of reduced catalytic activity and reduced tolerance to inhibitors, and achieve reduction of additional interference, strong inhibitor tolerance, The effect of high response sensitivity and accuracy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] The construction of embodiment 1M-MLV reverse transcriptase mutant
[0021] 1. Plasmid construction of reverse transcriptase mutant strains
[0022] According to the wild-type MMLV reverse transcriptase protein sequence (the gene sequence of the MMLV reverse transcriptase mother is shown in SEQ ID NO.1), after performing codon optimization suitable for Escherichia coli, insert it into the vector pBAD-hisA as the wild-type reverse transcriptase expression plasmid. According to the structural characteristics of reverse transcriptase, the mutation site is mainly selected:
[0023] (1) The structural domain involved in substrate binding and cDNA synthesis, mutating amino acids can improve the substrate binding ability;
[0024] (2) amino acids connecting the polymerase domain and the RNaseH domain, and mutating amino acids to improve its stability;
[0025] (3) RNase H domain, mutating amino acids to improve thermal stability or inhibitor tolerance and the ability to syn...
Embodiment 2
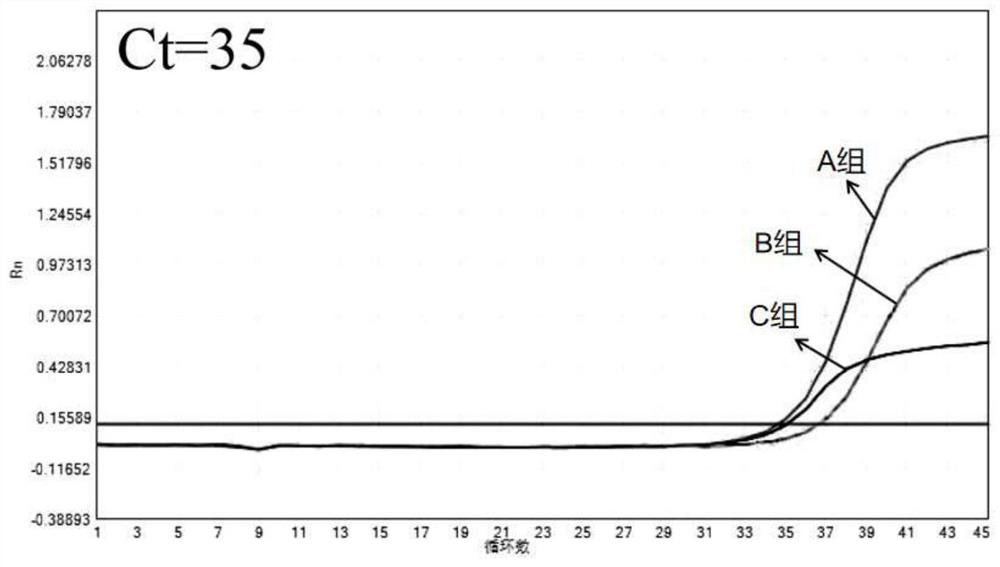
[0045] Embodiment 2M-MLV reverse transcriptase mutant performance verification
[0046] Perform performance evaluation on the obtained mutant reverse transcriptase, select clinical samples for detection, and set up 3 sets of reaction systems in total, and the 3 sets of reaction systems are all configured according to the reaction system described in Example 1, the difference is only in the reverse transcriptase, All the other components and consumption are exactly the same.
[0047] Group A: the reverse transcriptase is the reverse transcriptase described in the present invention;
[0048] Group B: the reverse transcriptase is Thermo Fisher's SuperScriptⅢ (SSⅢ);
[0049] Group C: the reverse transcriptase was Thermo Fisher's SuperScript IV (SS IV).
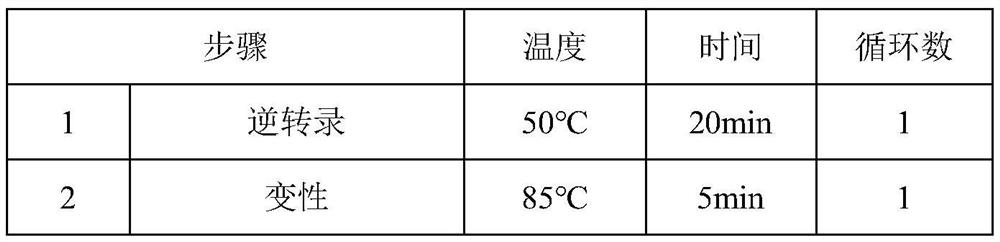
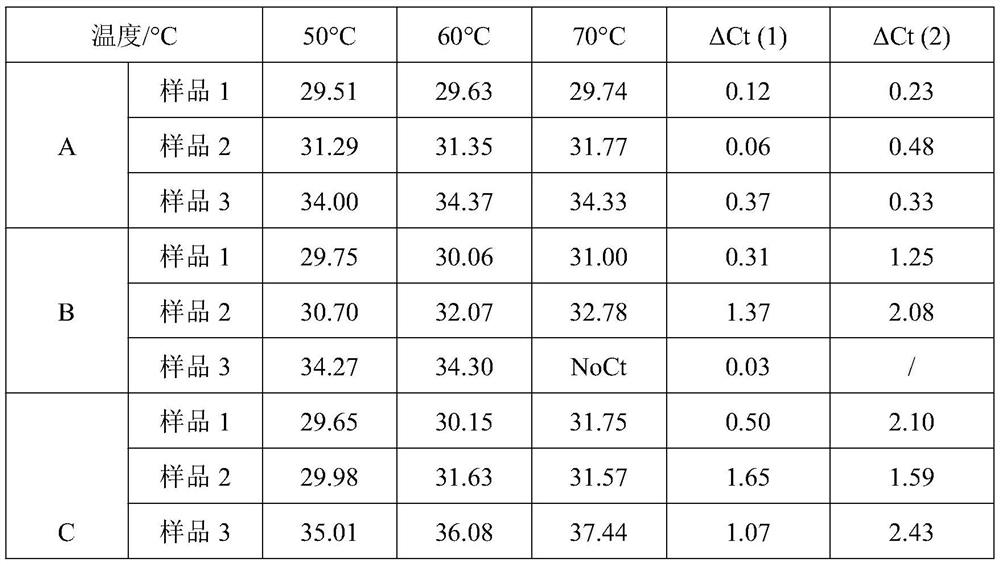
[0050] (1) Thermal stability verification of M-MLV reverse transcriptase mutants
[0051] In order to detect the thermal stability of reverse transcriptase, the above three groups of reaction systems were subjected to reverse t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com