Method for generating new faujasite zeolites
A technology of faujasite and size, applied in the field of sequential acid treatment and alkali treatment of faujasite, can solve the problems of reducing, insufficient to prepare attractive mesoporous faujasite, serious inherent zeolite properties and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0042] The present invention relates to a kind of preparation method of process catalyst, this method comprises the following steps:
[0043] - preparation of treated inorganic porous solids according to the first aspect of the present invention and preferred embodiments thereof;
[0044] - adding one or more additional ingredients to form the mixture, preferably wherein the one or more additional ingredients are selected from fillers, pyrogenic substances, binders, lubricants, and combinations thereof; and
[0045] - Shaping the mixture into a macroscopic form to obtain the process catalyst, preferably wherein the macroscopic form has a minimum dimension of at least 1 μm up to 10 cm.
[0046] The inventors have found that the solids obtained by the present invention are ideal intermediate compounds for the preparation of process catalysts as described above.
[0047] The mesoporous zeolite material obtained by the present invention can have controllable mesoporous volume, me...
Embodiment 10
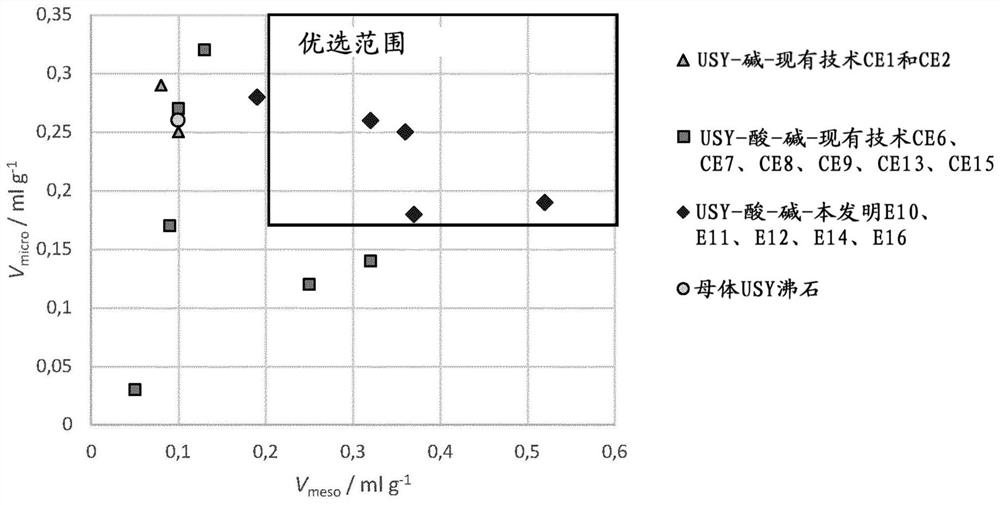
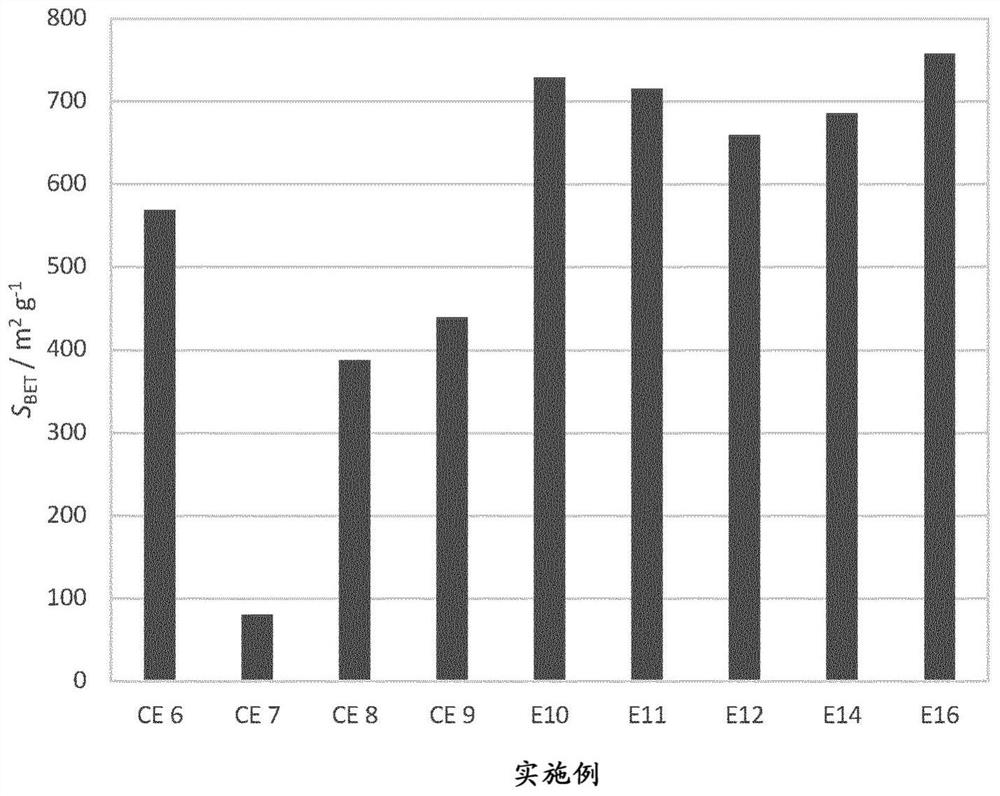
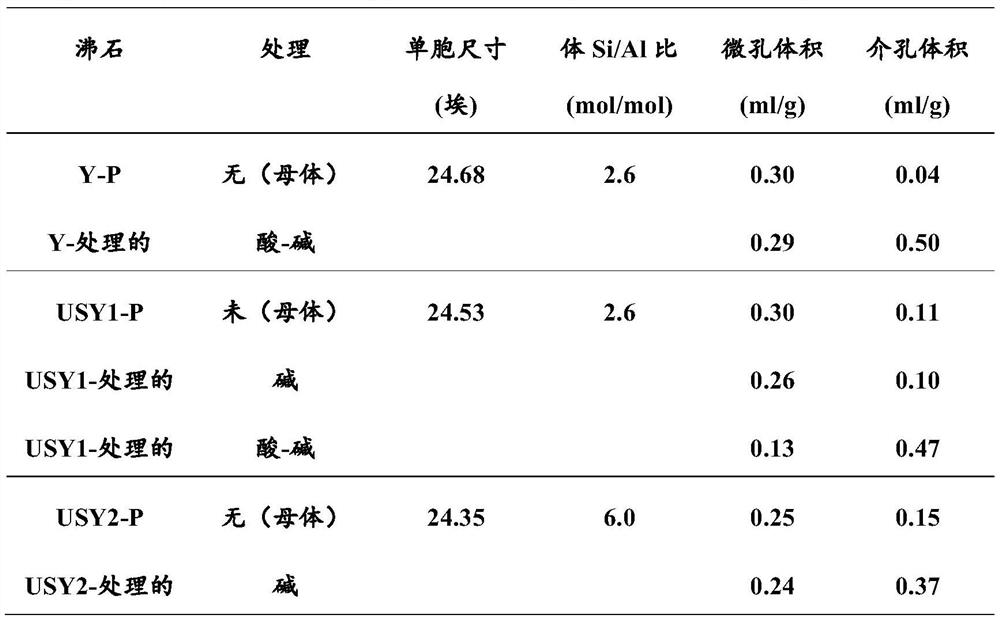
[0079] Example 10: In addition to using 2.2mmol g -1 NaH 2 Citrate instead of 1.6mmol g -1 Except for the citric acid, the same faujasite and treatment as in Comparative Example 6 were used. In this case, the amount of protons derived from the salt in the acid step was 50%. The solids obtained demonstrate that, like the solids obtained in Examples 11, 12, 14 and 16, the treatment of the invention applied to faujasite with Si / Al = 2.6 and a unit cell size of 24.53 Angstroms produces a BET with a significant increase mesoporous zeolites with largely preserved micropore volume and largely increased mesoporous surface and volume (Table 3). Therefore, only acid and base treatments according to the invention are able to produce octahedral zeolites with preferred porosity: high V micro and a high V meso ( figure 1 ) and high S BET ( figure 2 )s solid type. In each case of the examples, the amount of salt-derived protons in the acid step ranged from 20-90% at a pK range of -...
Embodiment 11
[0080] Example 11: In addition to using 1.1 mmol g -1 H 4 EDTA and 1.1 mmol g -1 Na 2 h 2 EDTA instead of 1.6mmol g -1 Except for the citric acid, the same faujasite and treatment as in Comparative Example 6 were used. In this case, the amount of protons derived from the salt in the acid step was 50%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com