Application of DADS in preparation of medicine for relieving intestinal flora disorder
A technology of intestinal flora and drugs, applied in the field of biomedicine, can solve problems to be studied and so on
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
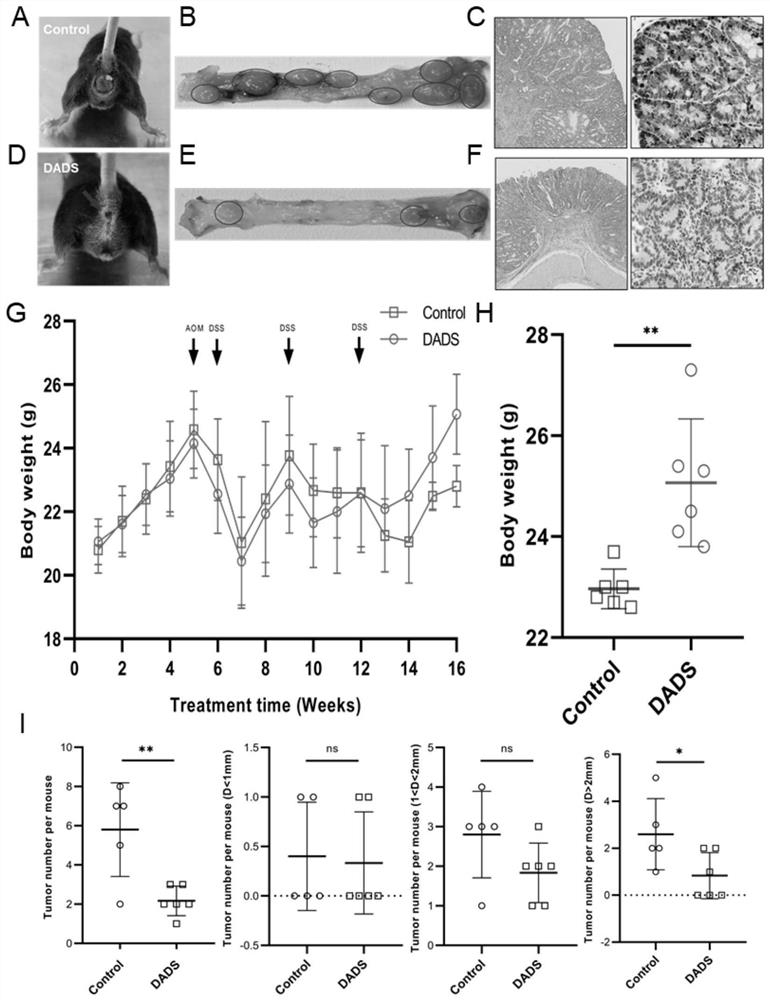
[0120] This embodiment verifies that DADS inhibits the occurrence and development of mouse CRC and studies its mechanism. The specific experimental methods and results are as follows:
[0121] 1. Experimental method
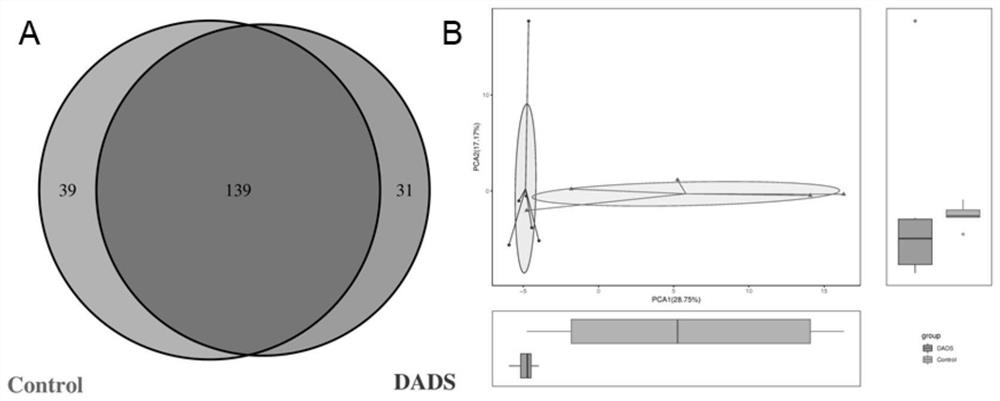
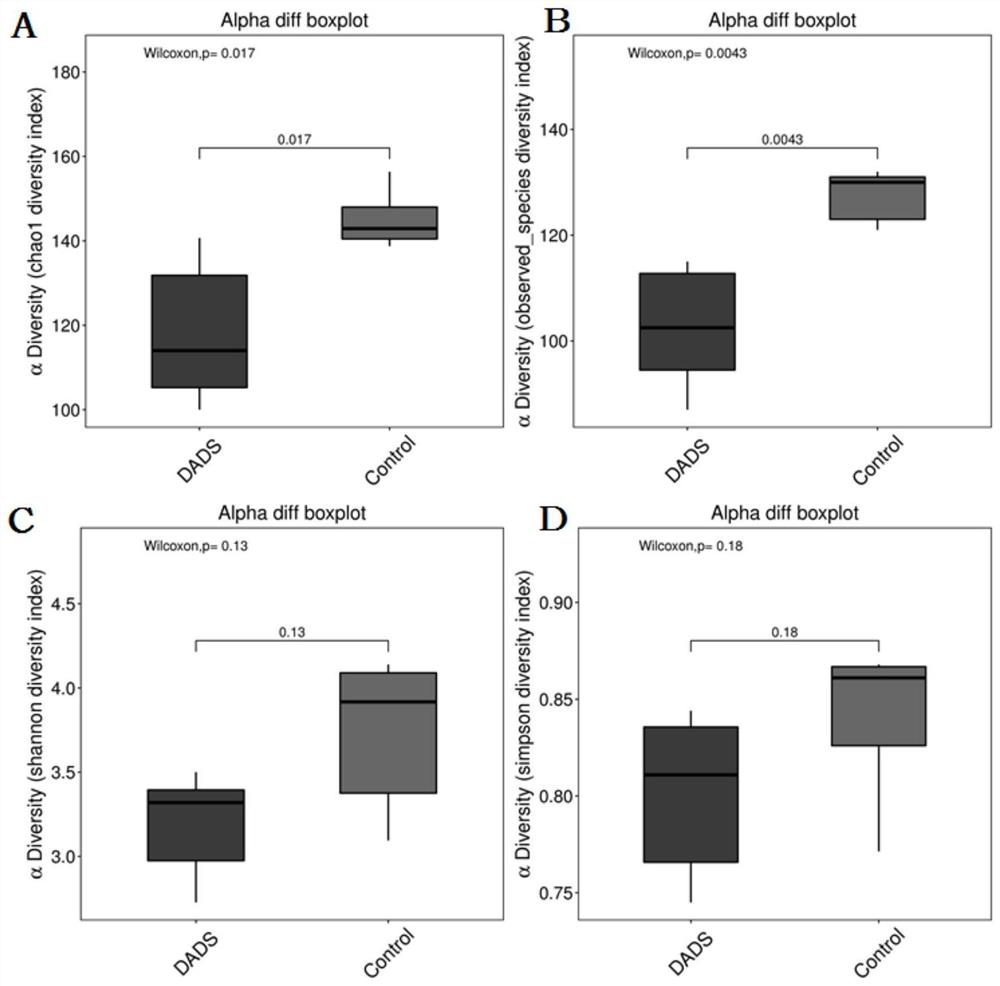
[0122] 11 C57BL / 6J mice were divided into control group (Control group, n=5) and DADS intervention group (DADS group, n=6), CRC tumors were induced by AOM / DSS at week 5, and DADS The control group continued to use the DADS diet intervention, while the control group used the AIG diet intervention, and the body weight of the animals was recorded every week. At the 16th week, animal feces were collected for 16S rDNA detection and metabolomics detection. Afterwards, the animals were sacrificed, and the intestinal tissues of the animals were removed for evaluation of tumor size and number. Part of the intestinal tissue was collected and fixed with neutral fixative, and the other part of the intestinal tissue was collected into cryopreservation tubes and stored in a -...
Embodiment 2
[0153] In this example, pseudo-sterile mice were used to verify that DADS lost its inhibitory effect on mouse CRC after the antibiotics disturbed the flora. The specific experimental methods and results are as follows:
[0154] 1. Experimental method
[0155] Ten 7-week-old healthy male C57B / L6 mice were randomly divided into a pseudo-sterile control group (Control_PGF group, n=5) and a pseudo-sterile DADS intervention group (DADS_PGF group, n=5), and they were fed adaptively for one week. The control group ate the AIG feed, and the intervention group ate the DADS feed. In the first 4 weeks, quadruple antibiotics were added to the drinking water of the two groups to establish a pseudo-sterile animal model. In the 5th week, the two groups of mice were combined with AOM / DSS to induce the intestinal cancer model, and the diet intervention of the two groups was as described above. Animals were sacrificed at week 16, and tissues were collected for detection.
[0156] 2. Results ...
Embodiment 3
[0173] In this example, by constructing fecal bacteria transplantation group animals, it is reversely verified that DADS cannot inhibit CRC in the absence of normal intestinal flora structure. The specific experimental methods and results are as follows:
[0174] 1. Experimental method
[0175] 1.1 Preparation of bacteria solution for fecal transplantation
[0176] 1) Collect about 20g of feces from healthy C57BL / 6J mice within 3 hours;
[0177] 2) Use physiological saline to prepare 10% glycerin solution, grind and mix with feces at a ratio of 5:1 after sterilization;
[0178] 3) The suspension was filtered through 2.0mm, 1.0mm, 0.5mm, 0.25mm aperture screens respectively to obtain 200mg / ml bacterial liquid;
[0179] 4) Collect the bacterial liquid and freeze it at -80°C.
[0180] 1.2 Ten 7-week-old healthy male C57 B / L6 mice were randomly divided into fecal microbiota transplantation control group (Control_FMTgroup, n=5) and fecal microbiota transplantation allicin interv...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com