Synthesis method of ivabradine hydrochloride key intermediate
A technology of ivabradine hydrochloride and synthesis method, which is applied in chemical instruments and methods, carboxylate preparation, carboxylate preparation and other directions, can solve problems such as high price
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
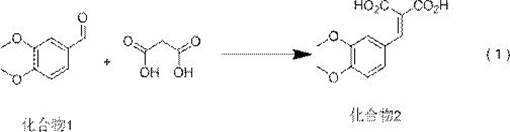
[0046] According to the reaction route of reaction formula (1)-(7), the first step: the reaction formula of reaction formula (1) is as follows:
[0047]
[0048] The method for preparing compound 2 in reaction formula (1) is as follows: take compound 1 (veratraldehyde), malonic acid, and catalyst 1 into the reaction bottle, add the reaction solvent of reaction formula (1), shake and dissolve, and set up the reaction Temperature and reaction time, heat to reflux for water separation reaction, after the reaction, cool down to room temperature and add water to stir, filter, wash with water, and dry to obtain compound 2. The specific reaction parameters are shown in the table below:
[0049]
[0050] Compound 2 1 H-NMR data: 1 H-NMR (400MHz, CDCl 3 ): δ: 3.98 (s, 3H), 3.99 (s, 3H), 7.46 (d, 1H), 7.70 (d, 1H), 7.90 (d, 1H), 8.32 (s, 1H).
[0051] Mass spectral data of compound 2: ESI+[M+H] + = 253.1
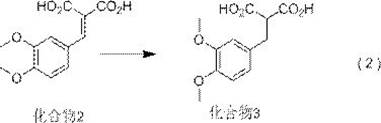
[0052] The second step: the reaction formula of reaction formula (2) ...
Embodiment 2
[0089] According to the synthesis method of Example 1, the difference is that the specific reaction parameters are changed.
[0090] The reaction parameters of reaction formula (1) are as follows:
[0091]
[0092] Compound 2 1 H-NMR data: 1 H-NMR (400MHz, CDCl 3 ): δ: 3.99 (s, 3H), 3.99 (s, 3H), 7.45 (d, 1H), 7.70 (d, 1H), 7.90 (d, 1H), 8.32 (s, 1H).
[0093] Mass spectral data of compound 2: ESI+[M+H] + =253.1
[0094] The reaction parameters of reaction formula (2) and reaction formula (3) are as follows:
[0095]
[0096] Compound 2 1 H-NMR data: 1 H-NMR (400MHz, CDCl 3 ): δ: 3.99 (s, 3H), 3.99 (s, 3H), 7.46 (d, 1H), 7.70 (d, 1H), 7.91 (d, 1H), 8.32 (s, 1H).
[0097] Mass spectral data of compound 4: ESI+[M+H] + =211.1
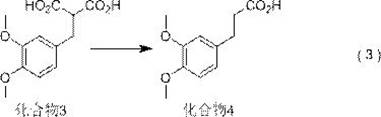
[0098] The reaction parameters of reaction formula (4) are as follows:
[0099]
[0100] Compound 5 1 H-NMR data: 1 H-NMR (400MHz, CDCl 3 ): δ: 7.02 (s, 1H), 6.78 (s,1H), 3.85 (s, 6H), 3.01 (t,2H), 2.69 (t,2H)
[0101] Mass spectra...
Embodiment 3
[0115] According to the synthesis method of Example 1, the difference is that the specific reaction parameters are changed.
[0116] The reaction parameters of reaction formula (1) are as follows:
[0117]
[0118] Compound 2 1 H-NMR data: 1 H-NMR (400MHz, CDCl 3 ): δ: 3.98 (s, 3H), 3.99 (s, 3H), 7.44 (d, 1H), 7.70 (d, 1H), 7.91 (d, 1H), 8.32 (s, 1H).
[0119] Mass spectral data of compound 2: ESI+[M+H] + =253.1
[0120] The reaction parameters of reaction formula (2) and reaction formula (3) are as follows:
[0121]
[0122] Compound 2 1 H-NMR data: 1 H-NMR (400MHz, CDCl 3 ): δ: 3.98 (s, 3H), 3.99 (s, 3H), 7.45 (d, 1H), 7.71 (d, 1H), 7.91 (d, 1H), 8.32 (s, 1H).
[0123] Mass spectral data of compound 4: ESI+[M+H] + =211.1
[0124] The reaction parameters of reaction formula (4) are as follows:
[0125]
[0126] Compound 5 1 H-NMR data: 1 H-NMR (400MHz, CDCl 3 ): δ: 7.01 (s, 1H), 6.78 (s,1H), 3.85 (s, 6H), 3.00 (t,2H), 2.68 (t,2H)
[0127] Mass spectra...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com