Organic room-temperature phosphorescent material as well as preparation method and application thereof
A room temperature phosphorescence, organic technology, applied in the field of preparation, organic room temperature phosphorescence materials, can solve problems such as restricting development and practical application
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
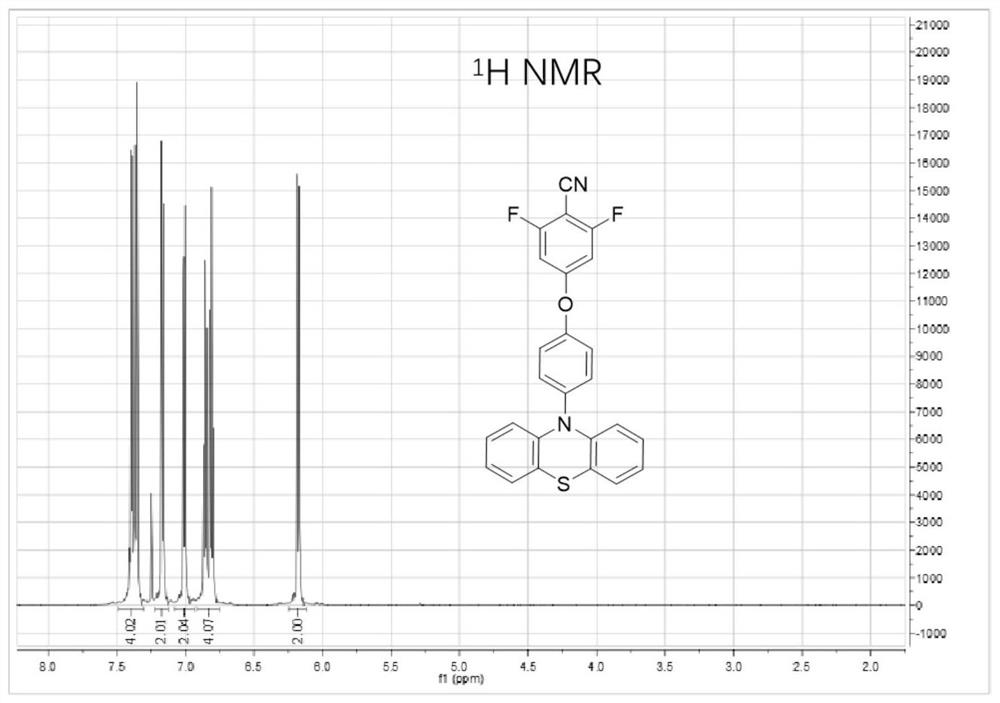
[0053] Embodiment 1: the synthesis of compound 2FCN-PTZ
[0054] Step 1: Synthesis of intermediate PTZ-Ar-OMe
[0055]
[0056] Add phenothiazine (5g, 25.1mmol), p-bromoanisole (7.1g, 30.2mmol), sodium tert-butoxide (3.62g, 37.7mmol) in turn into a 100mL reaction flask and then add 50mL of toluene solution until dissolved, Palladium acetate (1.13g, 5.03mmol) catalyst and tri-tert-butylphosphine (0.51g, 2.51mmol) were added thereto, and reacted at 110°C for 24h under a nitrogen atmosphere. After the reaction, add water to the reaction solution, extract 3 times with dichloromethane, dry with anhydrous magnesium sulfate after the organic phases are combined, concentrate under reduced pressure, separate by silica gel column chromatography (eluent is sherwood oil: dichloromethane =2:1), a white solid PTZ-Ar-OMe (5.2 g, yield: 67.9%) was obtained.
[0057] Step 2: Synthesis of intermediate PTZ-Ar-OH
[0058]
[0059] Add PTZ-Ar-OMe (5g, 16.4mmol) to the reaction flask and a...
Embodiment 2
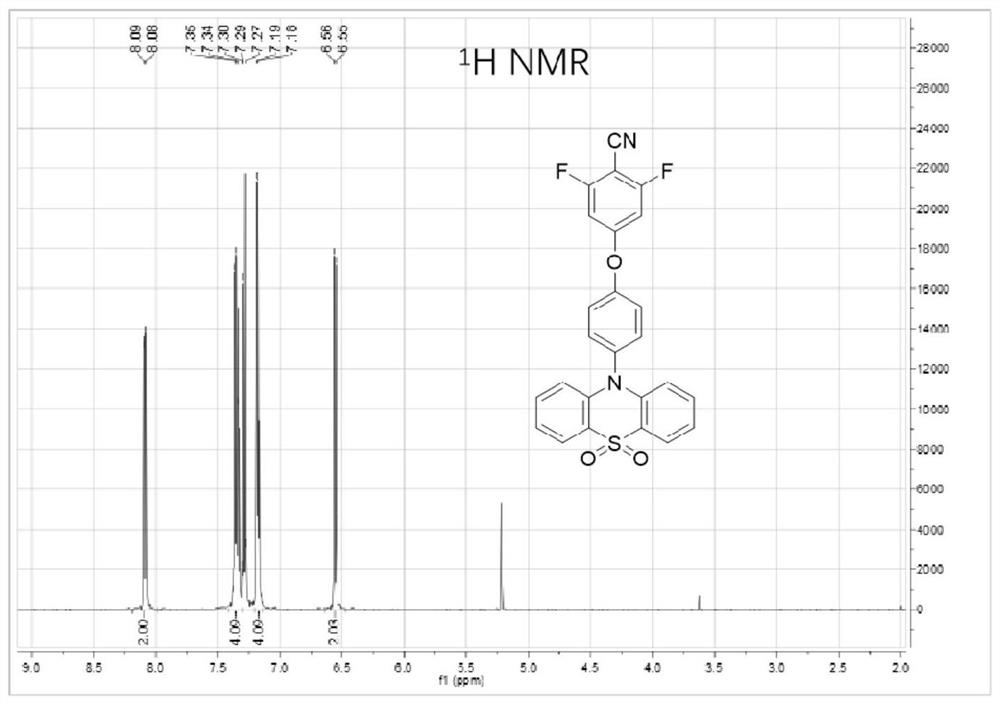
[0063] Embodiment 2: the synthesis of compound 2FCN-o-PTZSO
[0064]
[0065] The target product 2FCN-o-PTZ (1g, 2.3mmol) in Example 1 was dissolved in a mixture of dichloromethane (50mL), glacial acetic acid (25mL, 98%) and 30% aqueous hydrogen peroxide (1mL), and dissolved at 70 ℃ for 20 h, the reaction solution was extracted with dichloromethane, the organic phase was collected, and then anhydrous Na 2 SO 4 Dried and spin-dried to obtain the crude product. Using a mixture of petroleum ether and dichloromethane (v / v, 1:1) as an eluent, the crude product was separated and purified by silica gel column chromatography to obtain the target product 2FCN-o-PTZSO. The nuclear magnetic spectrum was as follows: figure 2 As shown, the yield was 56%.
Embodiment 3
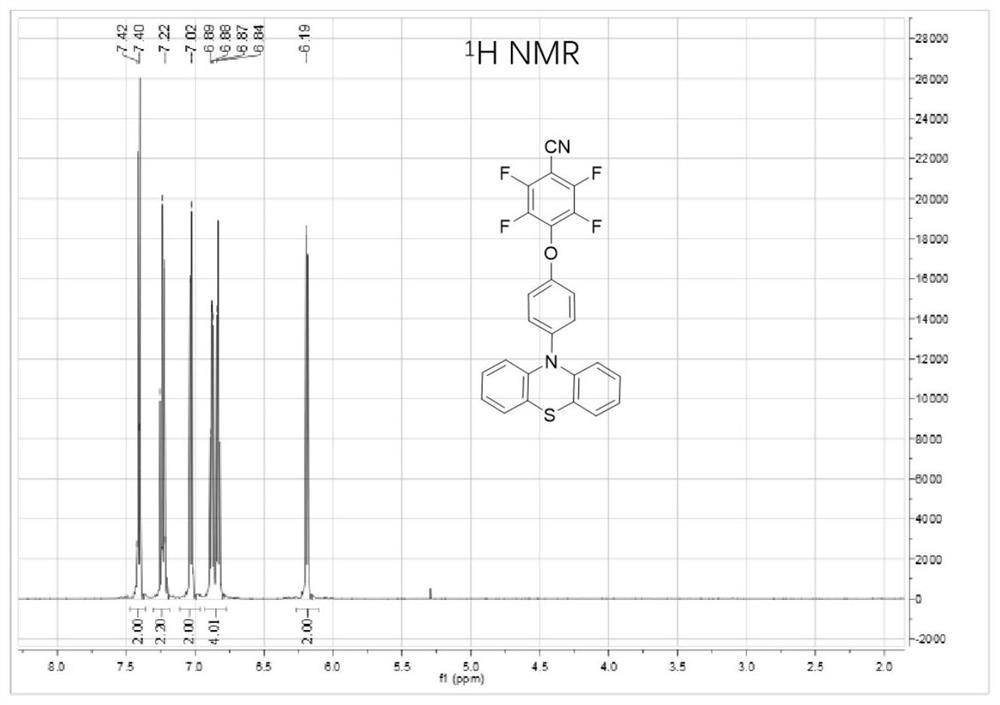
[0066] Embodiment 3: the synthesis of compound 4FCN-o-PTZ
[0067]
[0068] Using the same synthetic method as in Example 1, first react phenothiazine with p-bromoanisole to generate PTZ-Ar-OMe, then change the methoxyl group of the intermediate product into hydroxyl group and combine with 2,3,4,5,6 - pentafluorobenzene cyanide reaction, get final product 4FCN-o-PTZ, nuclear magnetic spectrum is as image 3 As shown, the yield was 67%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com