Method for preparing lithium iron manganese phosphate-carbon composite material and lithium iron manganese phosphate-carbon composite material
A technology of lithium iron manganese phosphate and carbon composite material, applied in structural parts, electrical components, battery electrodes, etc., can solve the problems of low specific capacity, high resistivity, poor cycle performance, etc., and achieve high specific capacity and low resistivity. , the effect of good conductivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
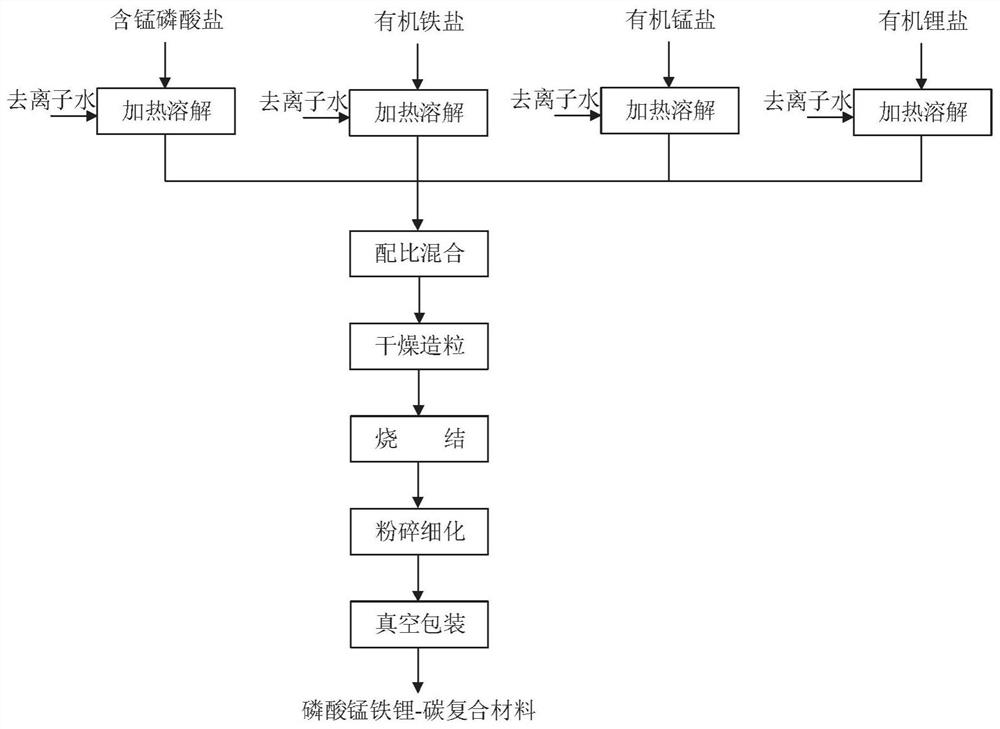
[0089] 1.1 Preparation of soluble manganese-containing phosphate solution
[0090]The soluble manganese-containing phosphate is added to deionized water according to the solute mass fraction of 10% to 60%, and phosphoric acid can be optionally added as a regulator of phosphorus content and pH value to prevent the formation of solid insolubles, phosphoric acid (according to pure The addition amount of phosphoric acid (calculated as phosphoric acid) does not exceed 15% of the mass of the manganese-containing phosphate, and the solution A is prepared.
[0091] The soluble manganese-containing phosphate may be manganese dihydrogen phosphate.
[0092] The phosphoric acid can be pure phosphoric acid or an aqueous phosphoric acid solution, such as concentrated phosphoric acid.
[0093] In this field, commonly used manganese sources can be divided into insoluble manganese sources and soluble manganese sources according to water solubility. Insoluble manganese sources mainly include m...
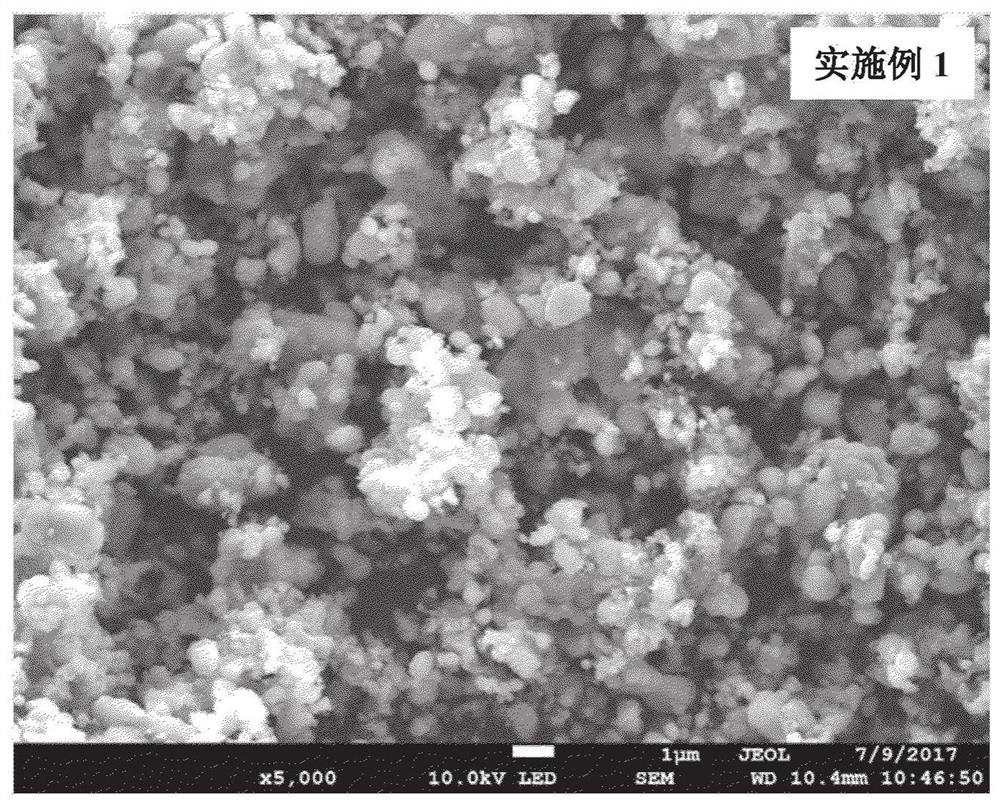
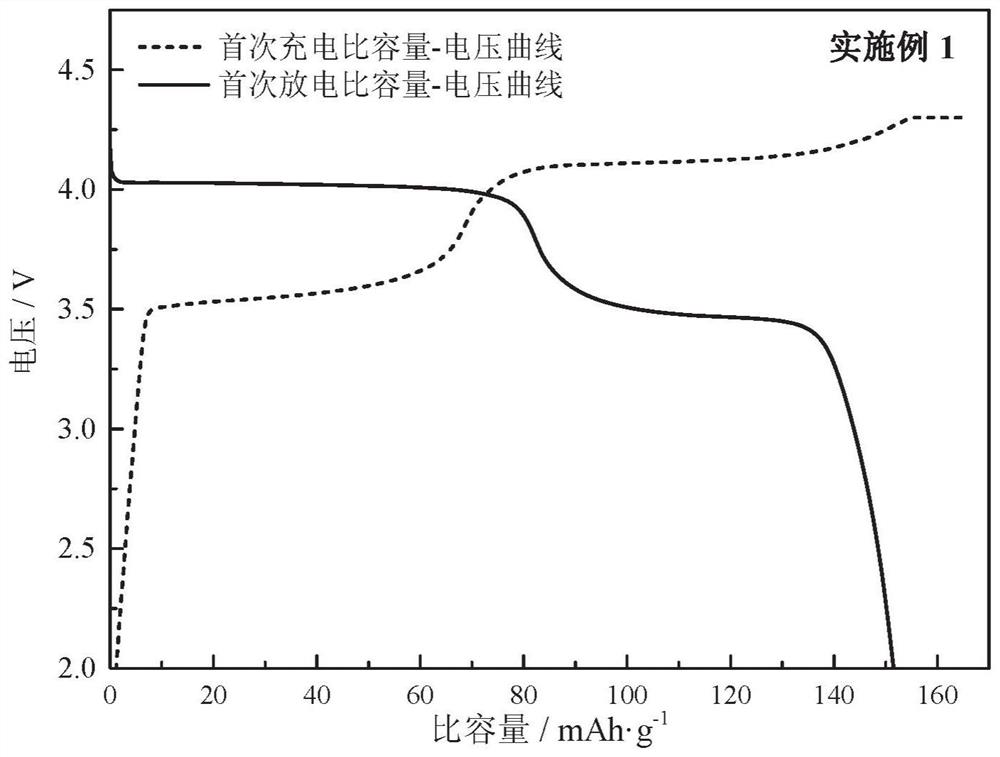
Embodiment 1
[0148] (1) Preparation of soluble salt solution
[0149] a. Weigh 27.92kg manganese dihydrogen phosphate dihydrate (wherein by mass, the Mn content is 15.01%, the P content is 21.90%, the same below), 1.44kg concentrated phosphoric acid (mass fraction is 85%) in 100kg deionized water , at a temperature of 45 °C, stirring and dissolving to obtain a manganese dihydrogen phosphate solution;
[0150] b. Weigh 28.00kg of ferric citrate pentahydrate (purity ≥ 99.5% by mass, the same below) in 60kg of deionized water, at a temperature of 30 ° C, stir and dissolve to obtain a ferric citrate solution;
[0151] c. Weigh 11.93kg of manganese acetate tetrahydrate (by mass, purity ≥99.5%, the same below) in 20kg of deionized water, stir and dissolve to obtain a manganese acetate solution;
[0152] d. Weigh 21.46kg of lithium acetate dihydrate (by mass, purity ≥99.5%, the same below) in 40kg of deionized water, stir and dissolve to obtain a lithium acetate solution;
[0153] (2) According...
Embodiment 2
[0158] The main difference between Example 2 and Example 1 is that ferrous lactate was used instead of ferric citrate pentahydrate as a soluble organic iron salt to prepare a lithium iron manganese phosphate-carbon composite material. details as follows:
[0159] (1) Preparation of soluble salt solution
[0160] a. Weigh 27.92kg manganese dihydrogen phosphate dihydrate, 0.46kg concentrated phosphoric acid (mass fraction is 85%) in 80kg deionized water, at a temperature of 65°C, stir and dissolve to obtain a manganese dihydrogen phosphate solution;
[0161] B. take by weighing 22.86kg ferrous lactate trihydrate (by mass, purity ≥ 99.5%, the same below), 1.14kg ascorbic acid in 200kg deionized water, at a temperature of 65 ℃, stirring and dissolving to obtain a ferrous lactate solution;
[0162] c. Weigh 10.39kg of manganese acetate tetrahydrate in 35kg of deionized water, stir and dissolve to obtain manganese acetate solution;
[0163] d. Weigh 22.27kg of lithium acetate dihy...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| electrical resistivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com