Injection inosine and preparation method thereof
A technology for injection and water for injection, which is applied in the field of medicine, can solve the problems of high risk of clinical use, fluctuation of shelf life of freeze-dried powder, unstable quality of finished products, etc., to avoid the introduction of other substances, high clarity, and stable quality Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
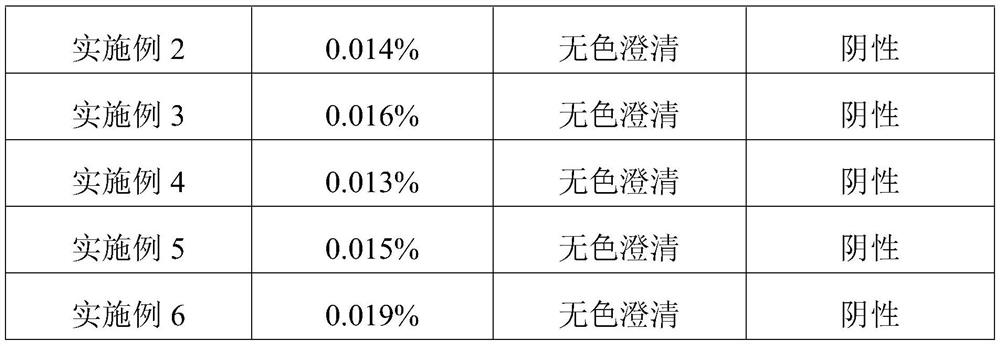
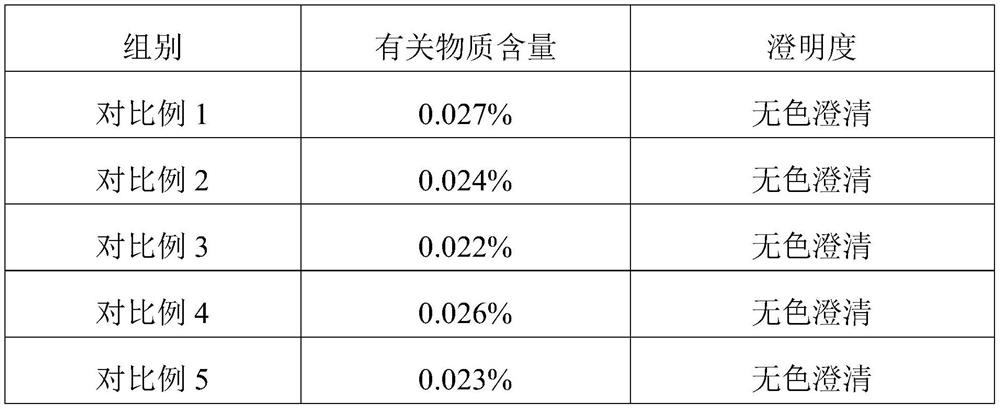
Embodiment 1
[0024] This embodiment provides a kind of inosine for injection, which is freeze-dried powder injection, each 0.2g, and its specific preparation method is as follows:
[0025] Dissolve NaCl in water for injection to prepare a solution with a concentration of 0.005mol / L, measure the pH of the solution to be 7.08, and set aside;
[0026] Add 200g of inosine to 4L of dissolving solution at a temperature of 10°C, keep the temperature constant during the dissolving process, then stir and slowly add 4g of sodium hydroxide until the inosine is completely dissolved to obtain a clear solution, and measure the pH of the clear solution Value is 12.46 (the initial pH of clarified liquid is in 11.5-13.1 and gets final product);
[0027] Add dropwise dilute hydrochloric acid with a concentration of 0.01mol / L to the clarified liquid to adjust the pH of the clarified liquid to between 8.0-9.0;
[0028] Add 250g of mannitol to the clarified liquid, stir until it is completely dissolved; after...
Embodiment 2
[0032] This embodiment provides a kind of inosine for injection, which is freeze-dried powder injection, each 0.2g, and its specific preparation method is as follows:
[0033] Dissolve NaCl in water for injection to prepare a solution with a concentration of 0.01mol / L, measure the pH of the solution to be 7.06, and set aside;
[0034] Add 200g of inosine to 4L of dissolving solution at a temperature of 20°C, keep the temperature constant during the dissolving process, then stir and slowly add 4g of sodium hydroxide until the inosine is completely dissolved to obtain a clear solution, and measure the pH of the clear solution The value is 12.33;
[0035] Add dropwise dilute hydrochloric acid with a concentration of 0.01mol / L to the clarified liquid to adjust the pH of the clarified liquid to between 8.0-9.0;
[0036] Add 250g of mannitol to the clarified liquid, stir until it is completely dissolved; after decolorization and impurity removal, filter, fill, and freeze-dry to obt...
Embodiment 3
[0039] This embodiment provides a kind of inosine for injection, which is freeze-dried powder injection, each 0.2g, and its specific preparation method is as follows:
[0040] Dissolve NaCl in water for injection to prepare a solution with a concentration of 0.05mol / L, measure the pH of the solution to be 7.02, and set aside;
[0041] Add 200g of inosine to 4L of dissolving solution at a temperature of 40°C, keep the temperature constant during the dissolving process, then stir and slowly add 4g of sodium hydroxide until the inosine is completely dissolved to obtain a clear solution, and measure the pH of the clear solution The value is 12.35;
[0042] Add dropwise dilute hydrochloric acid with a concentration of 0.01mol / L to the clarified liquid to adjust the pH of the clarified liquid to between 8.0-9.0;
[0043] Add 250g of mannitol to the clarified liquid, stir until it is completely dissolved; after decolorization and impurity removal, filter, fill, and freeze-dry to obt...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com