Specific chimeric antigen receptor cell targeting claudin18.2 and its preparation method and application
A chimeric antigen receptor, targeted binding technology, applied in genetically modified cells, cells modified by introducing foreign genetic material, animal cells, etc., can solve the problems of low tumor cell specificity and low safety, etc. To achieve the effect of high killing activity and high persistence
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
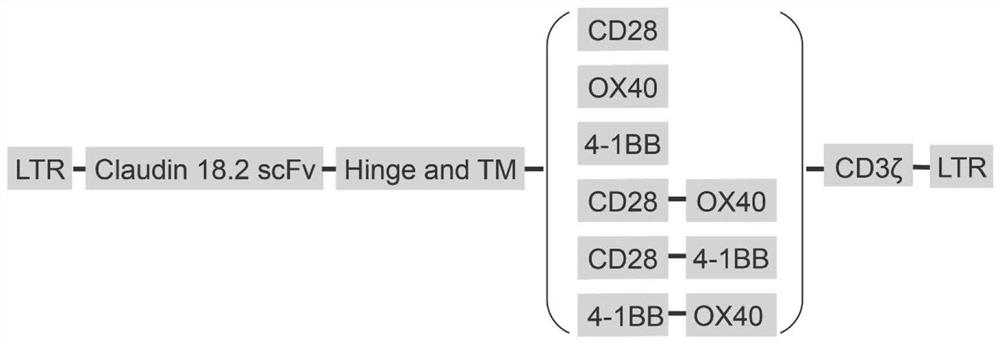
[0120] Example 1 Preparation of expression plasmids specifically targeting chimeric antigen receptors of human Claudin18.2
[0121] The overall design is as follows:
[0122] 1. Acquisition of anti-human Claudin18.2 single-chain antibody (scFv) gene sequence
[0123] The full human amino acid sequence number of Claudin18.2 in the Genbank database of the National Library of Medicine (NCBI) is: NP_001002026.1. After immunizing mice with human Claudin18.2 protein, its antibody fragment was purified and humanized to obtain a humanized single-chain antibody (scFv) against human Claudin18.2; the single-chain antibody consists of a light chain and a heavy chain, and the light chain The connecting segment between chain and heavy chain is (G4S) n (3≤n≤8), finally obtained 4 scFv antibody fragments with high affinity to the target antigen Claudin18.2 after effect screening, the amino acid sequence of which is as SEQ ID No.2 or SEQ ID No.3 or SEQ ID No.4 or Shown in SEQ ID No.5:
[0...
Embodiment 2
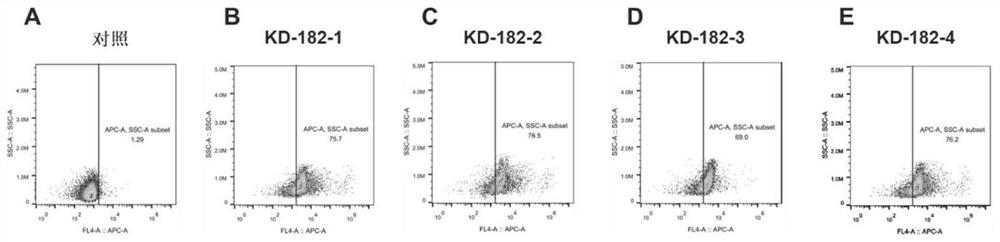
[0145] Example 2: Preparation of virus liquid of lentiviral vector
[0146] The recombinant plasmid expressing the chimeric antigen receptor specifically targeting human Claudin18.2 obtained in Example 1 and the packaging vectors psPAX2 and VSVG were used according to the ratio of 10:8:5 with Lipofectamine TM 6000 transfection reagent (purchased from ThermoFisher, product model 11668019) co-transfected 293T cells (see ThermoFisher transfection manual for specific transfection operation process), and replaced with complete medium 6 hours after transfection (purchased from Life Technologies , the product model is 11995-065), after 48 hours and 72 hours of culture, the cell supernatants rich in lentiviral particles were collected respectively, and the virus supernatants were concentrated by ultracentrifugation to obtain the expression-specific targeting human Claudin18. 2 chimeric antigen receptor lentiviral vector virus liquid (hereinafter referred to as KD-182-1 or KD-182-2 or...
Embodiment 3
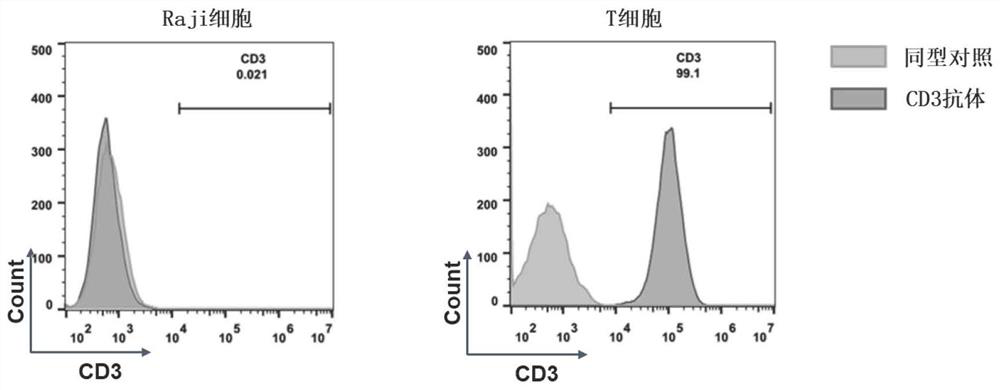
[0147] Example 3: Isolation and culture of T cells
[0148] Fresh peripheral blood from healthy donors was taken, and fresh peripheral blood mononuclear cells were separated by density gradient centrifugation; paramagnetic beads coupled with anti-CD3 antibody and anti-CD28 antibody (purchased from Invitrogen, USA, product information for Human T-Activator CD3 / CD28) to enrich CD3 + T cells, specifically, peripheral blood mononuclear cells were diluted to a concentration of (10-30) × 10 6 single cells / ml, and then mixed the magnetic beads and cells in a culture dish at a ratio of 3:1, incubated at room temperature for 2-3 hours, and used a Magnetic particles concentrator (MPC for short, purchased from Invitrogen, USA) company) to enrich CD3+ T cells. Finally, the enriched CD3+T cells were resuspended in culture medium (purchased from Life Technologies, USA, product information is OpTmizer TM T-Cell Expansion SFM), adjust the cell solubility to 1×10 6 pcs / ml, finally at 3...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com