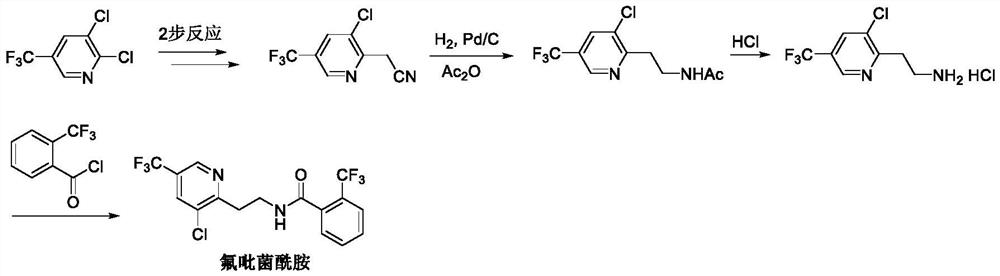
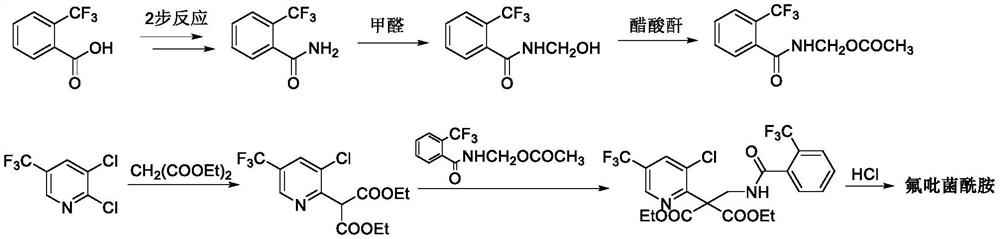
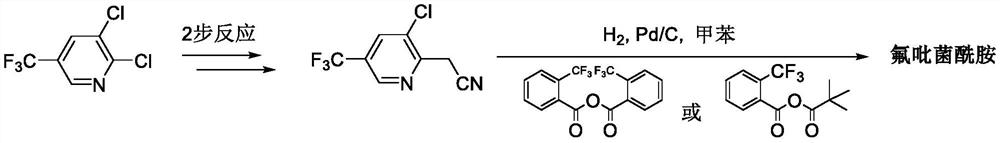
Method for synthesizing fluopyram
A technology for synthesizing fluopyram and fluopyram, applied in the fields of fine chemicals and organic synthesis, can solve problems such as pollution, and achieve the effects of high reaction yield, high product purity and short reaction route
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] 1) Add 205g of 2-bromoethylamine hydrobromide, 10L of 2-methyltetrahydrofuran and 404g of triethylamine into a 5L reaction flask, stir at 27°C, react until the raw materials are completely converted, place the reaction in an ice-water bath, Add 208 g of o-trifluoromethylbenzoyl chloride containing 1.1 L of 2-methyltetrahydrofuran solution dropwise, and the dropwise addition is completed in about 30 minutes. Remove the ice-water bath and continue the reaction until the conversion of cyclopropylamine is complete. Add 1.1 L of water to the reaction solution. Stir for 15 minutes, separate the layers, concentrate the organic phase, replace the organic phase with 2.5 L of petroleum ether, and filter to obtain cyclopropylamine-1-yl (2-(trifluoromethyl)phenyl)methanone.
[0035] The amount of cyclopropylamine-1-yl(2-(trifluoromethyl)phenyl)methanone obtained was 196 g, and the yield was 91.1%.
[0036] The 1H NMR of the product obtained in this step and 13 The C NMR spectrum d...
Embodiment 2
[0041] 1) Add 20.5g of 2-bromoethylamine hydrobromide, 300mL of dichloromethane and 40.4g of triethylamine into a 1L reaction flask, stir at 27°C, react until the raw materials are completely converted, place the reaction in an ice-water bath, Add dropwise a solution containing 110mL of dichloromethane solution and 20.8g of o-trifluoromethylbenzoyl chloride, and the dropwise addition is completed in about 10 minutes. Remove the ice-water bath and continue the reaction until the conversion of cyclopropylamine is complete. Add 110mL of water to the reaction solution. Stir for 15 minutes, separate the liquids, concentrate the organic phase, replace the organic phase with 300 mL of petroleum ether, and filter to obtain 20 g of cyclopropylamine-1-yl (2-(trifluoromethyl)phenyl)methanone, with a yield of 92.6%.
[0042] 2) Add 20g of 2,3-dichloro-5-trifluoromethylpyridine and 200mL of tetrahydrofuran into a 10L reaction flask, stir until completely dissolved, place in a low-temperatur...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com