Low-viscosity ketoconazole cream and preparation method thereof
A technology of viscosity ketoconazole cream and ketoconazole cream, which is applied in the directions of ointment delivery, pharmaceutical formulation, emulsion delivery, etc., can solve the problems of difficult filling and the cream is too viscous, etc., and achieves strong anti-yellowing ability Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] The screening of embodiment 1 prescription emulsifier
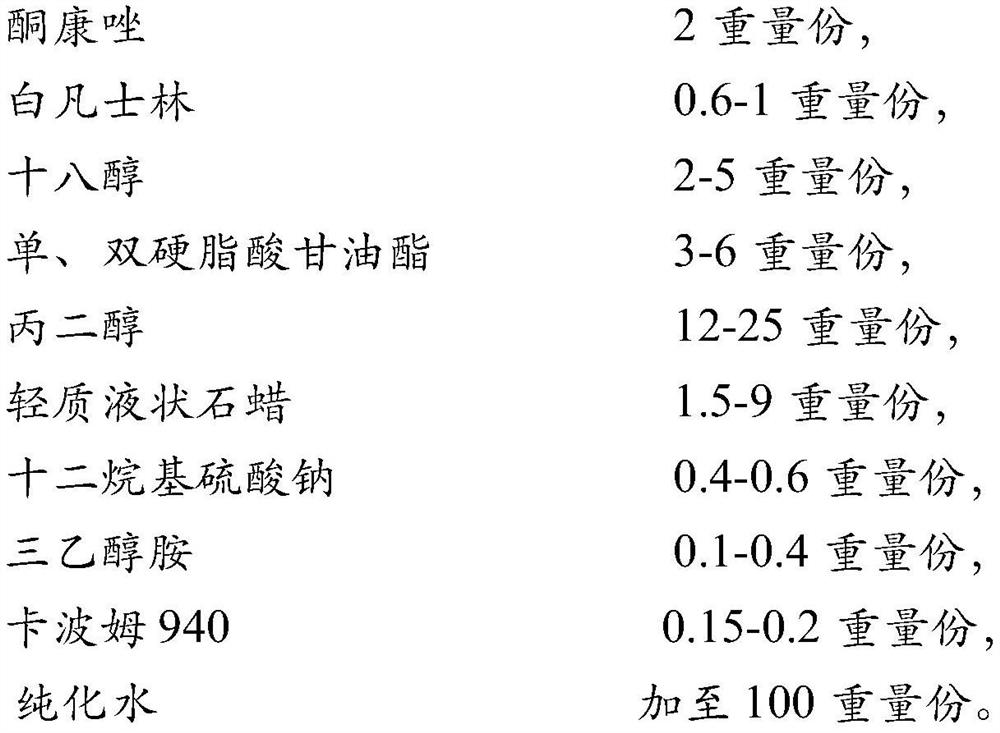
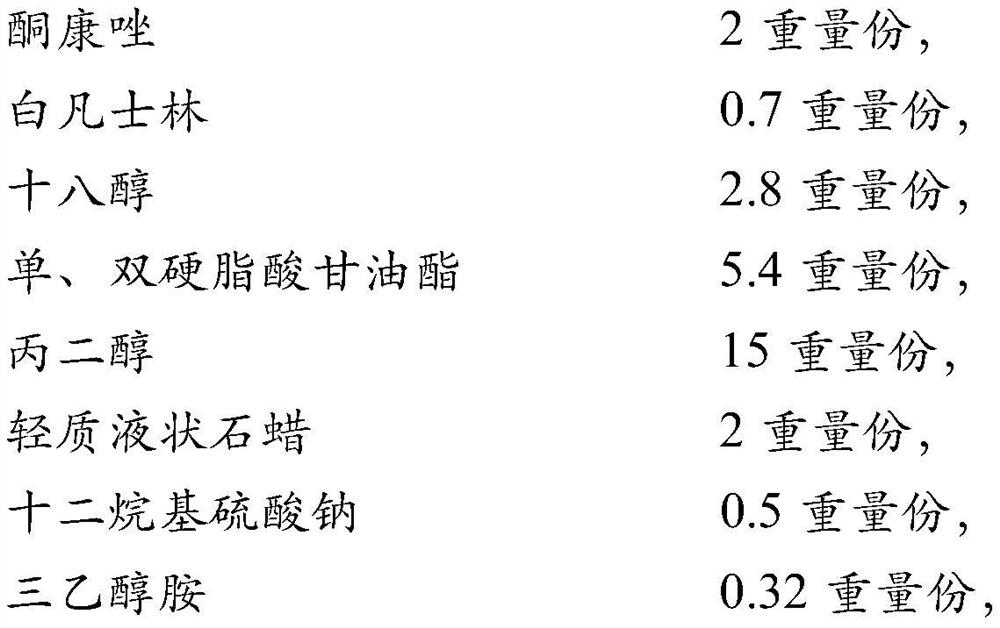
[0032] In order to improve the viscosity of ketoconazole cream and facilitate the filling in the workshop, the ratio of the matrix was further screened with reference to the prescription before the change. Design 4 prescriptions, as shown in Table 1 below, prepare the prescriptions in Table 1 according to the following process steps, and compare the appearance and viscosity with the prescription (prescription 5) before changing.
[0033] Preparation:
[0034] Heat white petrolatum, glyceryl mono- and distearate, stearic acid, stearyl alcohol, and ethylparaben in the prescribed amount to 75°C to 85°C, and use it as an oil phase to keep warm. Heat the prescribed amount of carbomer dispersion, 1 / 3 of the amount of glycerin (or 2 / 3 of the amount of propylene glycol), sodium lauryl sulfate, triethanolamine, and sodium sulfite to 80 ° C ~ 90 ° C, as a water phase to keep warm use. Under continuous stirring, add the oi...
Embodiment 2
[0042] The investigation of embodiment 2 prescription stability
[0043] In order to prevent delamination, the stability of this prescription 3, 4 is further investigated. Mainly investigate the tolerance of preparations to high temperature, low temperature and centrifugation.
[0044] The test method is as follows:
[0045] Heat-resistant test: Put the prepared cream samples into test tubes with stoppers and place them at a constant temperature of 60°C for 24 hours to observe whether there is liquefaction, delamination or discoloration.
[0046] Cold resistance test: Put each cream sample into a stoppered test tube and place it at a constant temperature of -15°C for 24 hours to observe whether there is liquefaction, delamination and discoloration.
[0047] Centrifugal test: put each cream sample into each centrifuge tube, and centrifuge for 30 minutes. Rotate at 3000 rpm, and observe whether there is separation.
[0048] The results of the stability test are shown in Tabl...
Embodiment 3
[0054] The screening of embodiment 3 prescription antioxidants
[0055] On the basis of prescription 4, further adjust the dosage of prescription antioxidant, design prescription 6-8, as shown in the following table 4, according to the preparation process of embodiment 1, prepare each prescription, and investigate the stability of each prescription.
[0056] The selection prescription design table of table 4 antioxidant
[0057]
[0058]
[0059] Stability study
[0060] According to the test conditions of influencing factors, the influence of adding different antioxidants on the preparation was mainly investigated under the conditions of high temperature 40°C and strong light (4500lx±500) for 10 days. Referring to the guiding principles of drug stability, samples were taken at 5 and 10 days to test the properties, stratification, uniformity, and content. The investigation results are shown in Table 5 below.
[0061] Table 5 Stability investigation results
[0062] ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| viscosity | aaaaa | aaaaa |
| viscosity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com