Alogliptin benzoate impurity as well as preparation method and detection method thereof
A detection method, the technology of benzoic acid, applied in the direction of measuring devices, instruments, scientific instruments, etc., can solve the problems of controlling the content of impurities, no impurities, and potential safety hazards of alogliptin benzoate, etc., achieving good repeatability and simple preparation process , the effect of good application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0058] Embodiment 1, the synthesis of alogliptin benzoate impurity (impurity I) of the present invention
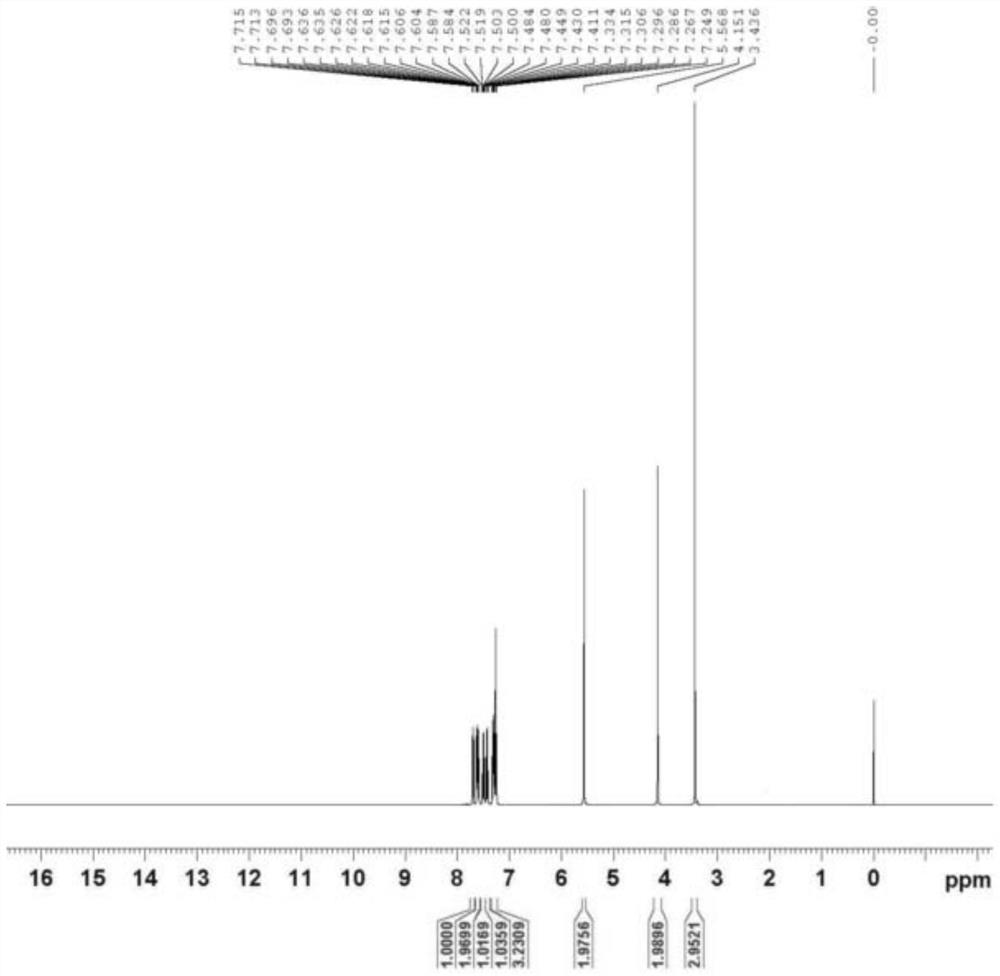
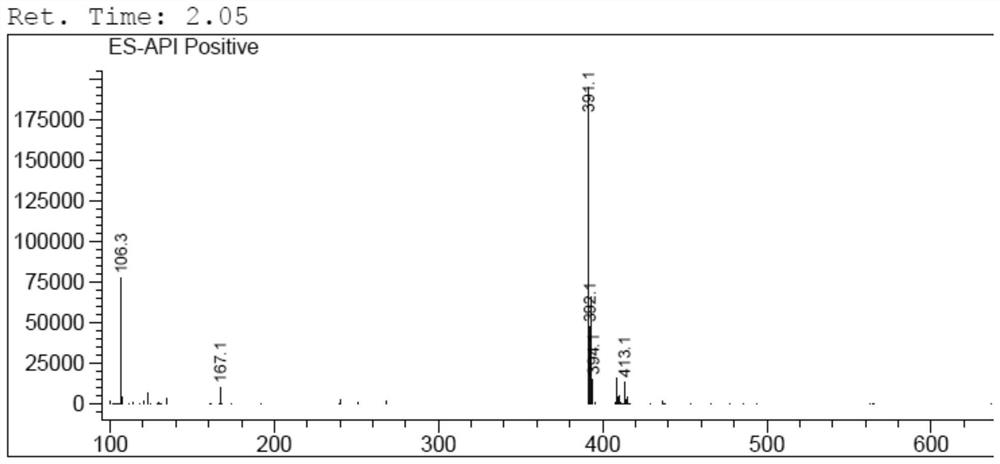
[0059]6-Chloro-3-methyluracil (3.2g, 0.02mol), 2-cyanobenzyl bromide (8.6g, 0.044mol), triethylamine (5.06g, 0.05mol) and N-methylpyrrolidone ( 20ml) into the reaction flask, heated to 80°C for 4.5h. Cool down to room temperature, add 30ml of water, and filter to obtain a crude product. Add 20ml of isopropanol, stir and beat at room temperature for 1h, filter, and dry to obtain impurity I (6.8g), with a yield of 87% and a purity of 97.2%. The data characterization spectrum of impurity I is as follows figure 1 and figure 2 As shown, the data characterization results are analyzed as follows:
[0060] 1 H-NMR (400MHz, CDCl 3 ):δ3.436(3H,s),4.151(2H,s),5.568(2H,s),7.249-7.334(3H,m),7.411-7.449(1H,m),7.480-7.522(1H,m ),7.584-7.636(2H,m),7.693-7.715(1H,m).
[0061] LCMS: m / z 391.1 [M+H] +
[0062]
Embodiment 2
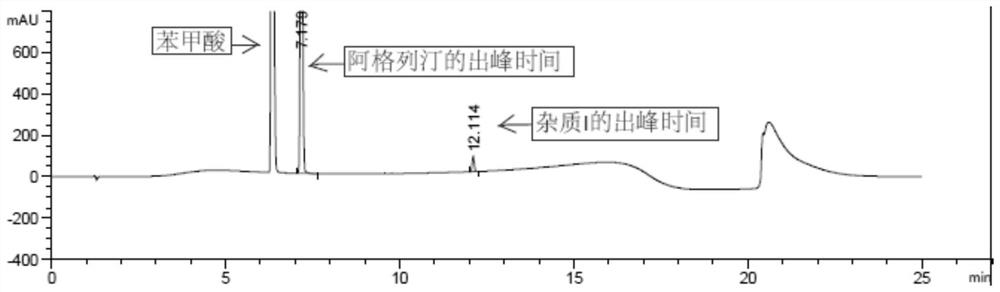
[0063] Embodiment 2, the impurity I prepared by the present invention is used as reference substance to carry out quality control research on alogliptin benzoate medicine
[0064] The alogliptin benzoate impurity (impurity I) prepared in Example 1 was used as a reference substance to carry out quality control research on alogliptin benzoate medicines.
[0065] Alogliptin benzoate is prepared according to the method described in WO2005095381A1.
[0066] The HPLC detection condition of alogliptin benzoate impurity of the present invention is as shown in table 1:
[0067] Table 1. The HPLC detection condition of alogliptin benzoate impurity of the present invention
[0068]
[0069] 1. The distinction between alogliptin benzoate and impurity I
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com