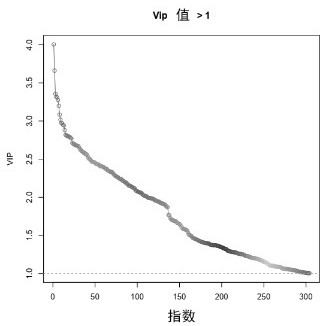
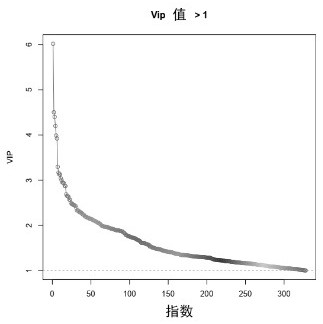
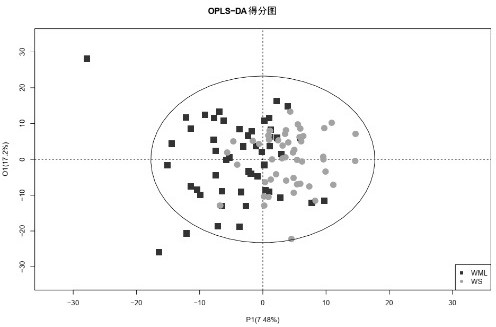
Biomarker for diagnosing cerebral infarction of white matter lesion patient and application of biomarker
A biomarker, cerebral infarction technology, applied in disease diagnosis, biological testing, biomaterial analysis, etc., can solve the problems of moving patients, high cost, unfavorable patient treatment and recovery, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] (1) Patient population standard
[0034] 1. The model building sample group is 102 people (internal population, which refers to the sample group used when building the prediction model)
[0035] Control group: 54 people with hyperintensity in brain white matter detected by MRI and no history of cerebral infarction, male to female ratio: 1:1, age range: over 45.
[0036] Patient population: 48 patients were sent to the hospital due to acute cerebral infarction due to MRI nuclear magnetic resonance examination showing high signal in the white matter of the brain. The male to female ratio: 1:1, and the age range: over 45.
[0037] 2. Model validation sample group 168 people (external population)
[0038] The sampling standard is the same as above.
[0039] Experimental equipment and reagents:
[0040] Sample collection: The serum of patients with only cerebral white matter lesions and cerebral infarction combined with white matter lesions were selected for testing.
[...
Embodiment 2
[0071] The logistic regression model was verified using an external data set, and the sampling standard was the same as in Example 1. This group provided 168 people, among whom hyperintensities appeared in the white matter of the brain, and 94 people who were sent to the hospital due to acute cerebral infarction showed hyperintensities in the white matter of the brain and anencephaly. 74 people with a history of infarction. Using the method in Example 1 to perform ultra-high performance liquid chromatography-mass spectrometry to detect the above 6 biomarkers, to verify the accuracy of the model results in Example 1, and draw the corresponding ROC curve, the results are as follows Show:
[0072] "Model A" variable is F1+F2, and the result of the ROC curve is as follows Figure 7 , Sensitivity (sensitivity)=1, Specificity (specificity)=1, Accuracy (accuracy)=1.
[0073] The "Model B" variable is F1+F3, and the results of the ROC curve are as follows Figure 8 As shown, Sensit...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com