Synthesis method of goserelin
A synthesis method, solid-phase synthesis technology, which is applied in the field of chemical synthesis of pharmaceutical polypeptide raw materials, can solve problems such as inability to synthesize yield, affect production cost, and low synthesis yield, so as to shorten the production cycle and reduce production cost Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0039]The technical solutions of the present invention are described in detail below in conjunction with the examples, and the present invention discloses a synthetic method of goserelin.
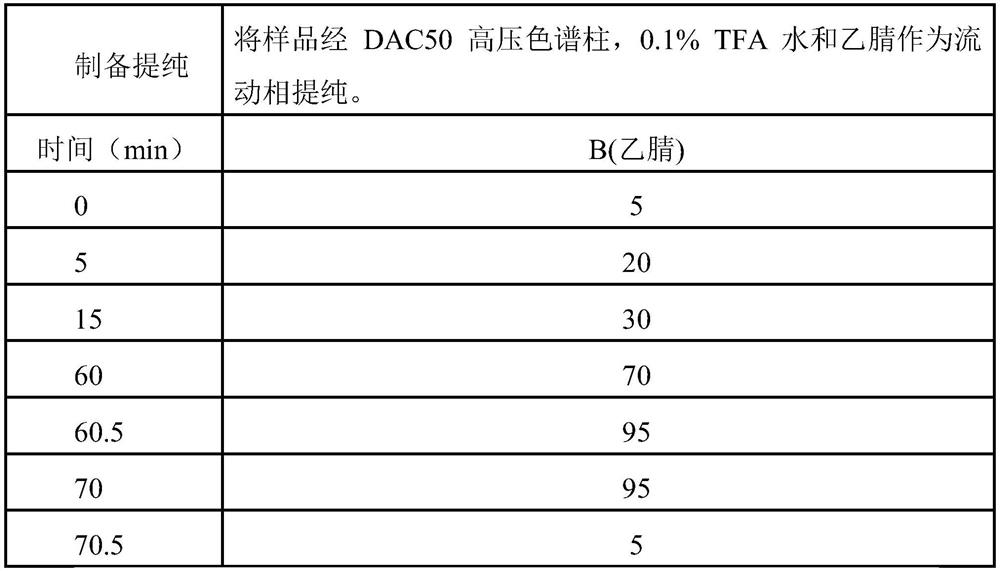
[0040] Weigh 20g of 2-CTC resin with a substitution degree of 1.5mmol / g, and place it in a solid-phase reaction column. DCM was swollen for 30min, and then a DMF solution of Fmoc-Tyr(tBu)-OH (45mmol) and DIEA (135mmol) was added to react fully for 4h. DMF was fully washed, and DCM:MeOH:DIEA=17:2:1 mixed solution was added to block for 15min. Washed 5 times with DMF to obtain Fmoc-Tyr(tBu)-CTC resin. According to the SPPS and sequence, Fmoc-Ser(tBu)-OH, Fmoc-Trp(Boc)-OH, Fmoc-His(Trt)-OH and Pyr-OH were coupled sequentially. Reaction with TFA:DCM=1:99 (volume ratio) mixed solution for 1h, rotary evaporation, beating with anhydrous ether, washing and drying to obtain the crude product of fully protected peptide (16.6g), through silica gel chromatography column: use 100ml of mixed solution D...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com