Synthesis method of fipronil intermediate
A cyano and dichloride technology is applied in the synthesis of pharmaceutical intermediates and in the field of synthesizing 5-amino-3-cyano-1-pyrazole disulfide, which can solve the problem that the finished product of fipronil does not meet the registration standard and increase the The process steps and the refining effect are unknown, and the effects of avoiding the discharge of bromine-containing wastewater, high purity and content, and reducing production costs are achieved.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
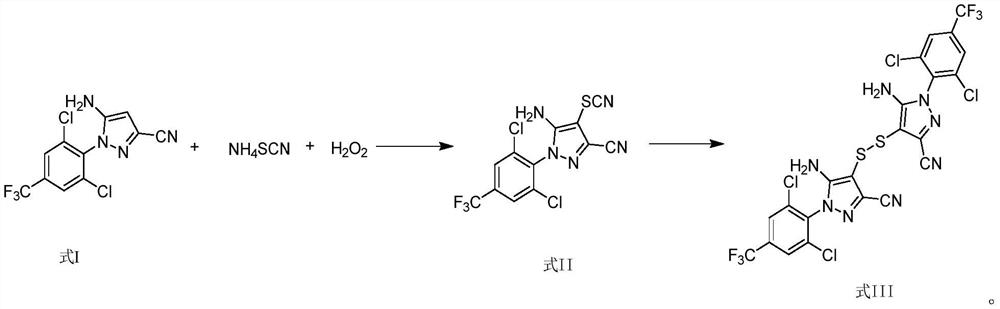
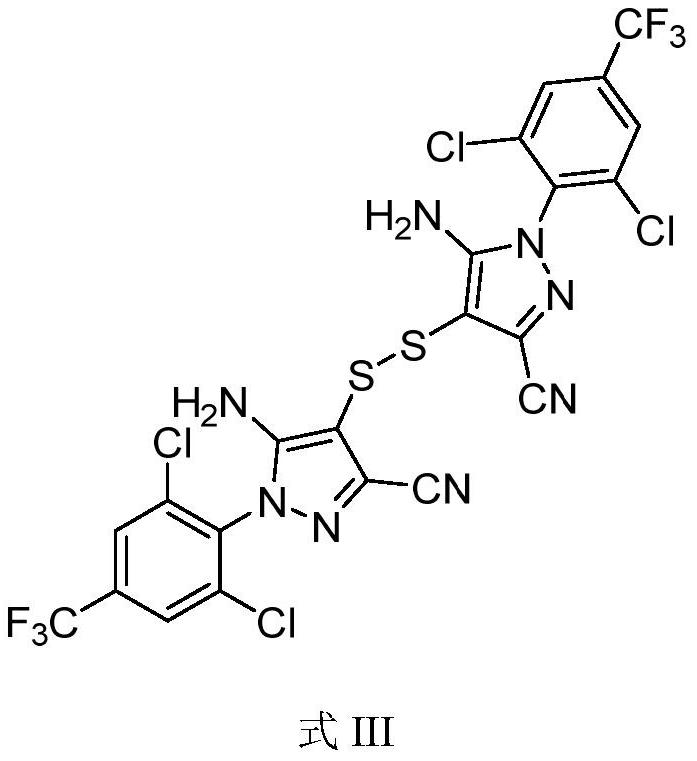
[0035] Add 200mL acetonitrile, 100g 5-amino-3-cyano-1-(2,6-dichloro-4-trifluoromethylphenyl)pyrazole (I) (0.31mol) and 59.3g Ammonium thiocyanate (0.78mol) was stirred, and 253g (3.72mol) of 50% hydrogen peroxide was added dropwise. The temperature of the reaction solution was controlled at -20°C to -15°C during the dropwise addition. Compound intermediate (II). Add 200 mL of water and adjust the pH to 9.0 with ammonia water. Raise the temperature to 80°C and stir for 1 hour. After the reaction is over, cool down to 0°C and keep it for 1 hour. Filter and dry to obtain 5-amino-3-cyano-1-(2,6-dichloro-4-trifluoromethyl-benzene Base) pyrazole disulfide (III) 102.1g, yield 93.1%, HPLC purity 99.80%, external standard content 98.9%.
Embodiment 2
[0037] Add 1500mL acetonitrile and 100g 5-amino-3-cyano-1-(2,6-dichloro-4-trifluoromethylphenyl)pyrazole (I) (0.31mol) and 94.5g Ammonium thiocyanate (1.24mol) was stirred, and 106g (1.55mol) of 50% hydrogen peroxide was added dropwise. The temperature of the reaction solution was controlled at 0°C to 10°C during the dropwise addition. Add 1500 mL of water and adjust the pH to 11.0 with ammonia gas. The temperature was raised to 50° C. for 3 hours, and the reaction was completed. Cool down to 5°C for 1 hour, filter and dry to obtain 5-amino-3-cyano-1-(2,6-dichloro-4-trifluoromethyl-phenyl)pyrazole disulfide (III) 98.7 g, yield 90.0%, HPLC purity 99.90%, external standard content 98.5%.
Embodiment 3
[0039] Add 500mL DMF and 100g 5-amino-3-cyano-1-(2,6-dichloro-4-trifluoromethylphenyl)pyrazole (I) (0.31mol) and 71.0g sulfur Ammonium cyanate (0.93mol) was stirred, and 169g (2.48mol) of 50% hydrogen peroxide was added dropwise. The temperature of the reaction solution was -10°C to 0°C during the dropwise addition. Add 500 mL of water and adjust the pH to 10.0 with ammonia gas. The temperature was raised to 70° C. for 1 hour, and the reaction was completed. Cool down to 5°C for 1 hour, filter and dry to obtain 5-amino-3-cyano-1-(2,6-dichloro-4-trifluoromethyl-phenyl)pyrazole disulfide (III) 105.0 g, yield 95.8%, HPLC purity 99.35%, external standard content 99.1%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com