Synthesis method of high-purity lornoxicam
A technology of lornoxicam and a synthesis method, which is applied to the synthesis field of high-purity lornoxicam, can solve the problems of decreased yield, increased reaction by-products, coking and the like, and achieves the effect of reducing the amount of solvent used
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
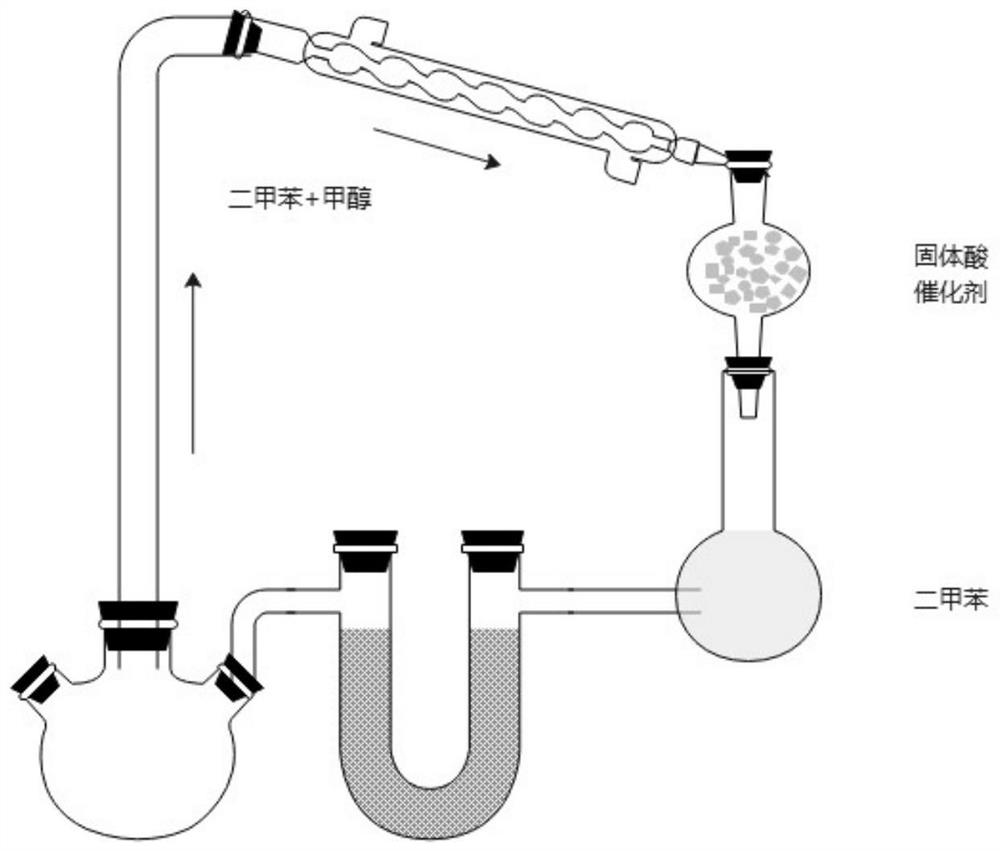
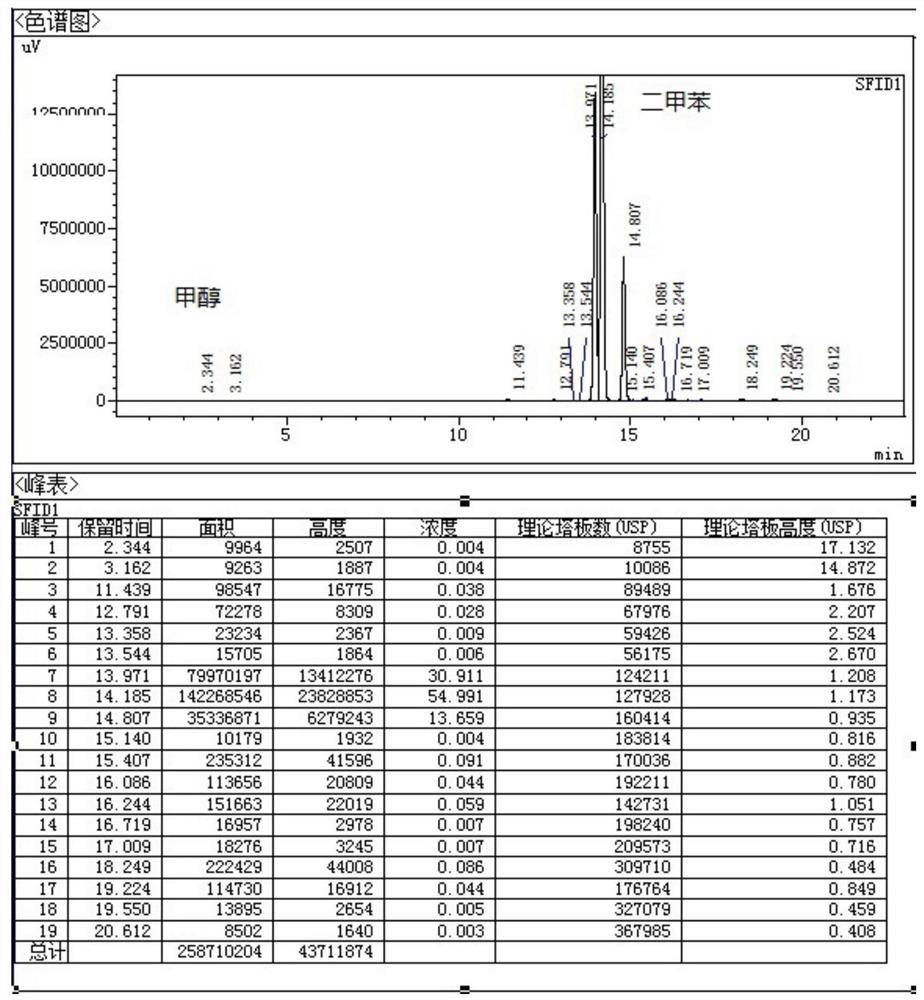
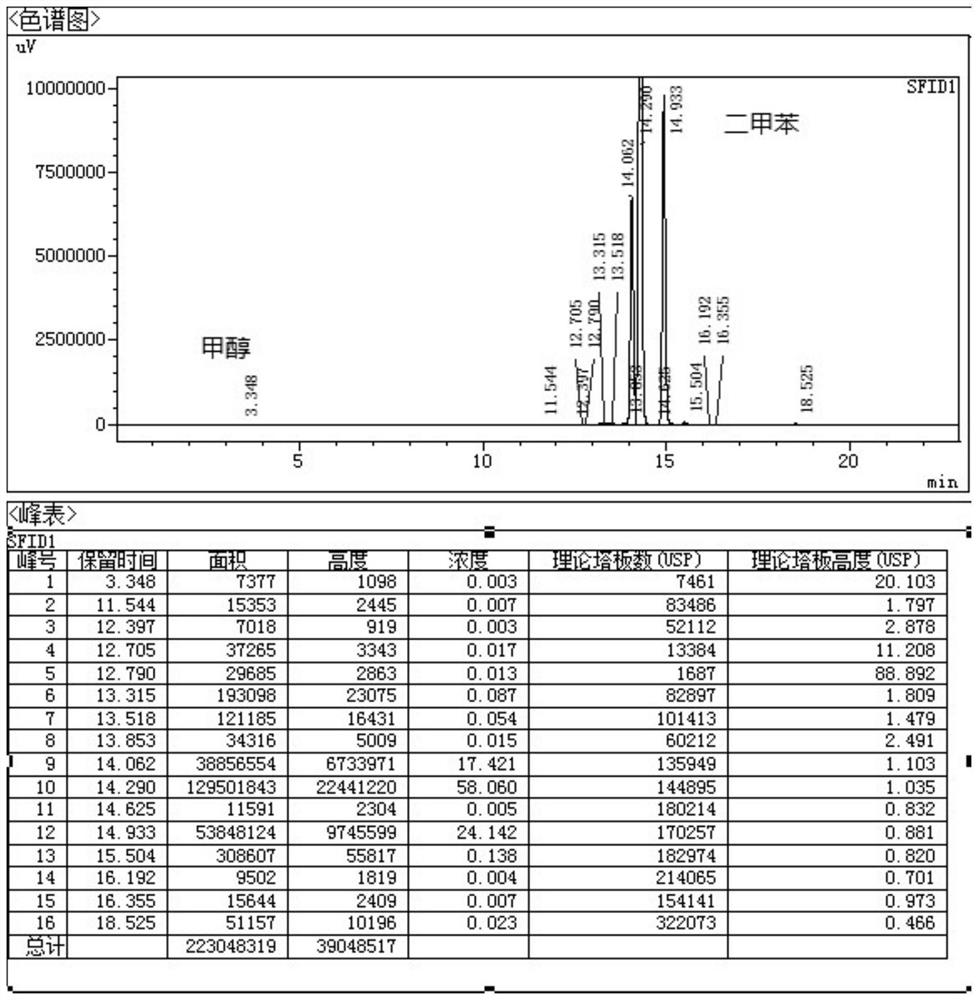
[0039] Under nitrogen protection, add 200ml of xylene, 6-chloro-4-hydroxy-2-methyl-2-H-thieno[2,3-e]-1,2-thiazinecarboxylic acid into a 500ml four-necked bottle 10g of methyl ester-1,1-dioxide and 3.65g of 2-aminopyridine were heated and distilled at 160°C. Using the device of the present invention, the methanol generated by the reaction boiled with xylene. The mixed gas of methyl alcohol that distillation reaction obtains and xylene is condensed and obtains condensate, enters and is equipped with 5g H 2 SO 4 / Fe 2 o 3In the solid superacid catalyst device, the methanol in the condensate is adsorbed, and the adsorbed xylene is refluxed to the reaction system through a U-shaped tube for recycling. The adsorption amount of methanol is 205mg / g. The reaction solution obtained by the distillation reaction was concentrated under reduced pressure, the reaction solution was concentrated to 100ml, cooled to room temperature, filtered to obtain the crude lornoxicam, and then refined ...
Embodiment 2
[0042] Under nitrogen protection, add 200ml of xylene, 6-chloro-4-hydroxy-2-methyl-2-H-thieno[2,3-e]-1,2-thiazinecarboxylic acid into a 500ml four-necked bottle 20g of methyl ester-1,1-dioxide and 7.3g of 2-aminopyridine were heated and distilled at 120°C. Using the device of the present invention, the methanol generated by the reaction boiled with xylene. The mixed gas of methyl alcohol and xylene obtained by the distillation reaction is condensed to obtain a condensate, which enters a tank containing 20g H 2 SO 4 / TiO 2 In the solid superacid catalyst device, the methanol in the condensate is adsorbed, and the adsorbed xylene is returned to the reaction system through a U-shaped tube for recycling. The adsorption amount of methanol is 314mg / g. The reaction solution obtained by the distillation reaction was concentrated under reduced pressure, the reaction solution was concentrated to 100ml, cooled to room temperature, filtered to obtain the crude lornoxicam, and then refin...
Embodiment 3
[0045] Under nitrogen protection, add 300ml of xylene, 6-chloro-4-hydroxy-2-methyl-2-H-thieno[2,3-e]-1,2-thiazinecarboxylic acid into a 500ml four-necked bottle 20g of methyl ester-1,1-dioxide and 7.3g of 2-aminopyridine were heated and distilled at 140°C. Using the device of the present invention, the methanol generated by the reaction boiled with xylene. The mixed gas of methyl alcohol that distillation reaction obtains and xylene is condensed and obtains condensate, enters and is equipped with 40g H 2 SO 4 / ZrO 2 In the solid superacid catalyst device, the methanol in the condensate is adsorbed, and the adsorbed xylene is refluxed to the reaction system through a U-shaped tube for recycling. The adsorption amount of methanol is 300mg / g. The reaction liquid obtained by the distillation reaction was concentrated under reduced pressure, the reaction liquid was concentrated to 150ml, cooled to room temperature, filtered to obtain the crude lornoxicam, and then refined with 1,...
PUM
| Property | Measurement | Unit |
|---|---|---|
| adsorption capacity | aaaaa | aaaaa |
| adsorption capacity | aaaaa | aaaaa |
| adsorption capacity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com