Pharmaceutical composition for controlling glucose fluctuation
A technology for blood sugar fluctuation and composition, which is applied in the directions of drug combination, pharmaceutical formula, medical preparation containing active ingredients, etc., and can solve the problems such as the listing of drugs with blood sugar fluctuation.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
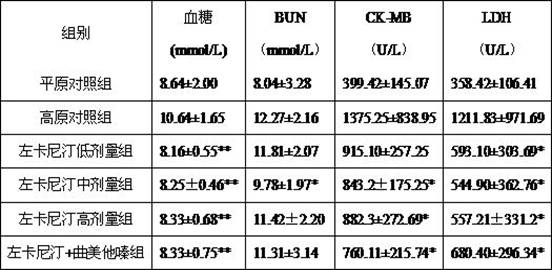
[0017] Example 1: Effects on Stress Increase in Blood Glucose Caused by High Altitude Hypoxia
[0018] 72 male SD rats were randomly divided into: plain control group, plateau control group, levocarnitine 200mg / kg, 400mg / kg, 600mg / kg groups, levocarnitine 600mg+ trimetazidine 3mg / kg, each Group of 12. Rats in the plain control group were fed in a normal plain environment, and 1 hour after the last administration in the plain environment of the other groups, the rats were placed in a low-pressure hypoxic animal experiment cabin, and the pressure was 6m·s. -1 Reduce blood pressure, rise to a simulated altitude of 6000m, administer the drug by gavage in the cabin for 2 days, and drop the simulated altitude to 5000m during the administration, deprive of water and food for the last 12 hours in the cabin, and maintain a simulated altitude of 5000m for 2 hours after the last dose, and press 1.5g / kg intraperitoneal injection of 10% urethane for anesthesia, fixed in supine position, ...
Embodiment 2
[0024] Example 2: Effects on exercise-induced hypoglycemia
[0025] Kunming mice, 40, were randomly divided into blank control group, L-carnitine 300mg / kg group, 600mg / kg group and 900mg / kg group. 10ml / kg intragastric administration, 2 times a day, continuous administration for 7 days. From day 6 to day 7, fast for 12 hours. 30 minutes after the last drug administration, the mice swam with a 3% load for 30 minutes, rested for 5 minutes, and took blood to measure blood glucose and blood lactic acid levels.
[0026] The results showed that the blood sugar of the mice decreased significantly after fasting exercise, and the L-carnitine intervention group had a significantly higher blood sugar and a significantly lower blood lactic acid content than the blank control group.
[0027] group Blood sugar (mmol / L) Blood lactic acid (mmol / L) Blank control group 3.12±0.54 2.32±0.49 L-carnitine low dose group 5.72±0.43** 2.28±0.69 L-carnitine middle dose ...
Embodiment 3
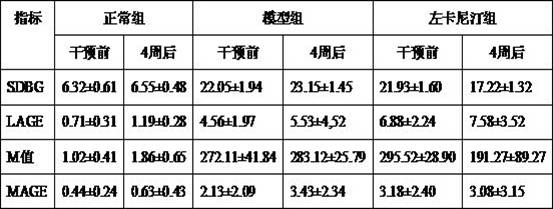
[0029] Example 3: Effects on Blood Sugar Fluctuation in High Glucose Model Rats
[0030] Thirty SD rats were randomly divided into normal group, model group and levocarnitine group, 10 in each group. Preparation of animal high-glucose model: Rats were fed with high-fat and high-sugar diet for 6 weeks, fasted for 12 hours, and STZ was injected intraperitoneally at a dose of 30 mg / kg. Modeling was successful when random blood glucose > 16.7 mmol / L and remained stable for 1 week. The levocarnitine group was given 400 mg / kg intragastric administration, and the normal group and model group were given the same volume of normal saline, once a day, for 4 consecutive weeks.
[0031] Observation indicators:
[0032] 1. Intraperitoneal glucose tolerance (IPGTT), area under the curve (AUCg)
[0033] The IPGTT experiment was carried out in accordance with the IPGTT operation method established by the American Diabetes Complications Animal Model Association (AMDCC): ①Rats were strictly f...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com