Asymmetric synthesis method of trifluoromethyl-containing multifunctional cyclopentenone derivative
A technology of trifluoromethyl and cyclopentenone, applied in the field of asymmetric synthesis of polyfunctionalized cyclopentenone derivatives, achieves mild reaction conditions, high chemical yield, enantioselectivity and diastereoselectivity Excellent effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
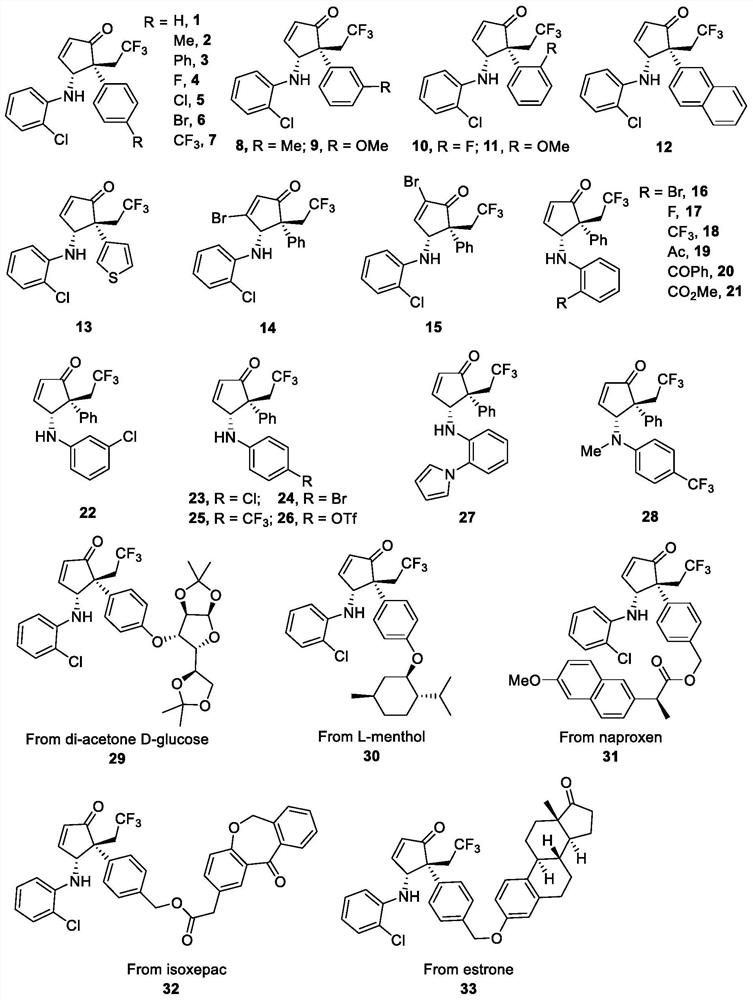
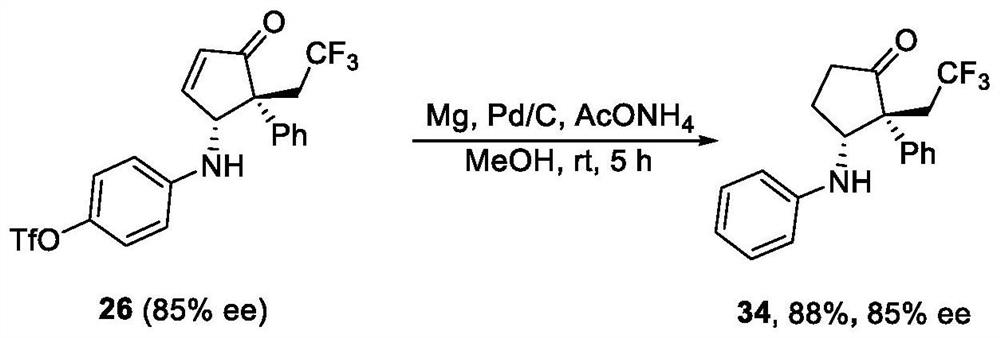
Examples
Embodiment 1
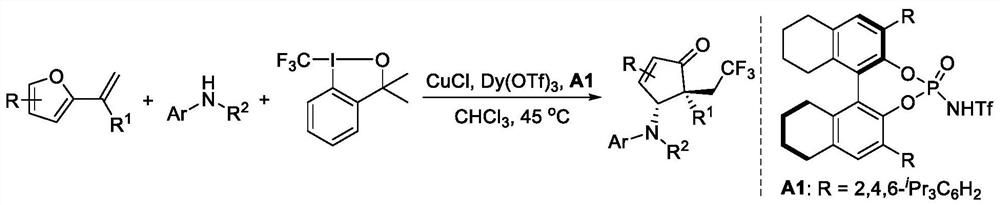
[0026] (4R,5R)-4-((2-chlorophenyl)amino)-5-phenyl-5-(2,2,2-trifluoroethyl)cyclopent-2-ene-1- The synthetic route of ketones:
[0027]
[0028] The reaction catalyst CuCl (0.5mg, 0.005mmol), Dy (OTf) 3 (1.2mg, 0.002mmol) and Bronsted acid A1 (5.4mg, 0.006mmol) were placed in a reaction tube, and a magnetic stir bar of appropriate size was placed. Vacuum-dried, replaced with argon three times, and added 4 mL of chloroform under the protection of argon. The reaction was then cooled to 0°C, and Togni reagent 3a (49.5mg, 0.15mmol), o-chloroaniline (0.10mmol, 1.0eq) and 2-(1-phenylvinyl)furan (0.15mmol, 1.5eq) were added sequentially ). The temperature was raised to 45°C and stirred at this temperature, the reaction was monitored by TLC. After the reaction was completed, after flash column chromatography, 1,3,5-trimethoxybenzene was used as an internal standard to carry out 1 H NMR characterization to determine the NMR yield and dr value of the product. The crude product wa...
Embodiment 2
[0032] (4R,5R)-4-((2-Chlorophenyl)amino)-5-(4-tolyl-5-(2,2,2-trifluoroethyl)cyclopent-2-ene-1- Ketones (2):
[0033]
[0034] With 2-(1-(p-tolyl)vinyl)furan (0.15mmol, 1.5eq), 2-chloroaniline (0.1mmol, 1.0eq) and Togni reagent 3a (49.5mg, 0.15mmol) as the reaction raw materials, the rest Operation is with embodiment 1. After reacting for 12 hours, it was purified by silica gel column chromatography (petroleum ether / ethyl acetate=20 / 1–15 / 1) to obtain product 2 in the form of yellow oil, with a NMR yield of 90%, an isolation yield of 83%, and >20 / 1dr . HPLC analysis yielded 92%ee (Chiralcel OD, hexane / i-PrOH=90 / 10, flow rate=1.0mL / min, l=254nm), t r (major) = 8.0 min, t r (minor) = 6.2min; [α] D 20 =–112.5 (c=0.97, in CHCl 3 ). 1 H NMR (500MHz, CDCl 3 )δ7.66–7.56(m,1H),7.20–7.12(m,2H),7.08(d,J=7.8Hz,2H),6.90(d,J=8.4Hz,2H),6.85(d,J =8.2Hz,1H),6.65(t,J=7.5Hz,1H),6.62–6.57(m,1H),5.27(d,J=10.4Hz,1H),3.88(d,J=10.4Hz,1H ),3.24–3.05(m,2H),2.30(s,3H). 13 C NMR (126MHz, CD...
Embodiment 3
[0036] (4R,5R)-5-([1,1'-biphenyl)-4-yl)-4-((2-chlorophenyl)amino)-5-(2,2,2-trifluoroethane Base) cyclopent-2-en-1-one (3):
[0037]
[0038] With 2-(1-(p-tolyl)vinyl)furan (0.15mmol, 1.5eq), 2-chloroaniline (0.1mmol, 1.0eq) and Togni reagent 3a (49.5mg, 0.15mmol) as the reaction raw materials, the rest Operation is with embodiment 1. After reacting for 24 hours, it was purified by silica gel column chromatography (petroleum ether / ethyl acetate=20 / 1–15 / 1) to obtain the product 3 as a white solid, with a NMR yield of 87%, an isolation yield of 82%, and >20 / 1dr . HPLC analysis yielded 93%ee (FLM Chiral INB, hexane / i-PrOH=90 / 10, flow rate=1.0mL / min, l=254nm), t r (major)=42.1min,t r (minor) = 9.9min; [α] D 20 =–29.2 (c=0.97, in CHCl 3 ); mp=138–140°C. 1 H NMR (500MHz, CDCl 3 )δ7.68–7.62(m,1H),7.52(d,J=7.5Hz,2H),7.47(d,J=7.0Hz,2H),7.43(t,J=7.5Hz,2H),7.35( t,J=7.5Hz,1H),7.18(t,J=8.0Hz,1H),7.13(d,J=7.2Hz,1H),7.06(d,J=7.1Hz,2H),6.88(d, J=8.2Hz, 1H), 6.69–6.63(m, 2H), 5.3...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Separation yield | aaaaa | aaaaa |
| Separation yield | aaaaa | aaaaa |
| Separation yield | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com