Polypeptide and application thereof in preparation of immunomodulatory drugs
A drug and composition technology, applied in the field of polypeptide and its application in the preparation of immunomodulatory drugs, can solve the problems of scarcity of polypeptide immunomodulatory drugs and low safety of immunomodulatory drugs, and achieve small molecular weight, easy preparation, and stable good sex effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0087] Example 1, PEP20 sequence analysis and in vitro synthesis
[0088] 1. PEP20 sequence analysis
[0089] The sequence of the host gene of LINC 01871 is as follows (SEQ ID NO: 1):
[0090]
[0091]
[0092]
[0093] In the above sequence, the underlined part in bold is the nucleotide sequence of PEP20. The sequence translated into amino acids is: MVEEIQASLMWQQAREREGE (SEQ ID NO: 2).
[0094] 2. In vitro synthesis of PEP20
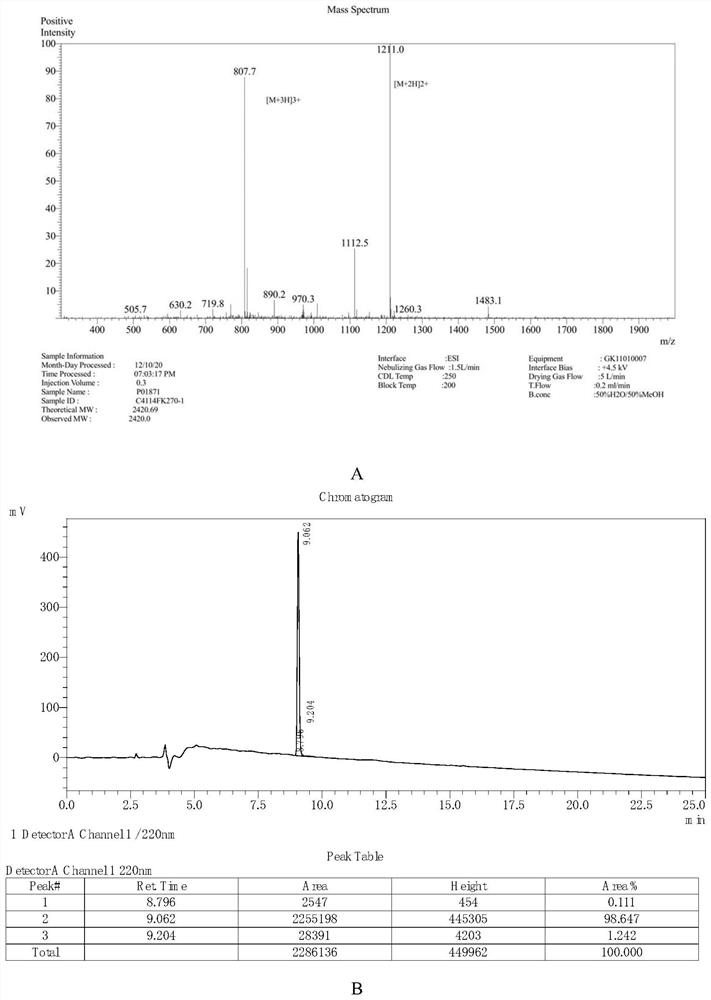
[0095] The conventional solid-phase peptide synthesis method is used to synthesize the peptide according to the amino acid sequence of SEQ ID NO: 2, and the correctness and purity of the obtained peptide are analyzed by mass spectrometry and HPLC detection. The result is as figure 1 As shown, the mass spectrometry analysis confirmed that the amino acid was correct, and its molecular weight was 2420.69 ( figure 1 A) of A); The purity of the obtained polypeptide detected by HPLC is 98.65% ( figure 1 B). Dissolve in HBSS before use.
Embodiment 2
[0096] Example 2, PEP20 inhibits Tc17 cell differentiation
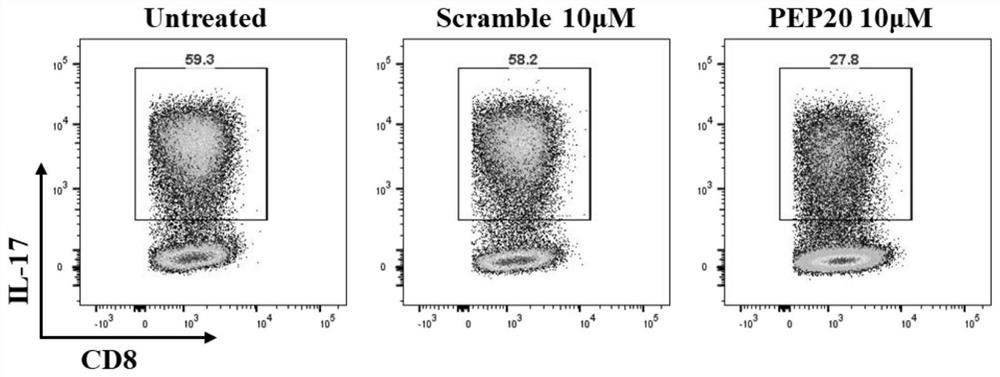
[0097] The PEP20 obtained by the solid-phase polypeptide synthesis method in Example 1 was tested for its effect on the differentiation of Tc17 cells.
[0098] Obtain mouse spleen cells, and use immunomagnetic beads to isolate mouse naive CD8 + T cells were cultured in RPMI-1640 medium at 37°C, and anti-CD3 (5 μg / ml), anti-CD28 (2 μg / ml), TFG-β (10 ng / ml), IL -23 (20 ng / ml), anti-IL-4 (5 μg / ml) and anti-IFN-γ (10 μg / ml) were cultured for 3 days to obtain Tc17 cells.
[0099] Tc17 cells were cultured in RPMI-1640 medium at 37°C and divided into three culture groups: Untreated group, Scramble group and PEP20 group, in which PEP20 group was added with 10 μM concentration of PEP20, and Scramble group was added with 10 μM concentration of Scramble Peptides (randomly scrambled sequences with the same 20 amino acids as PEP20), the Untreated group did not receive any treatment. The effect of PEP20 on Tc17 differentiation ...
Embodiment 3
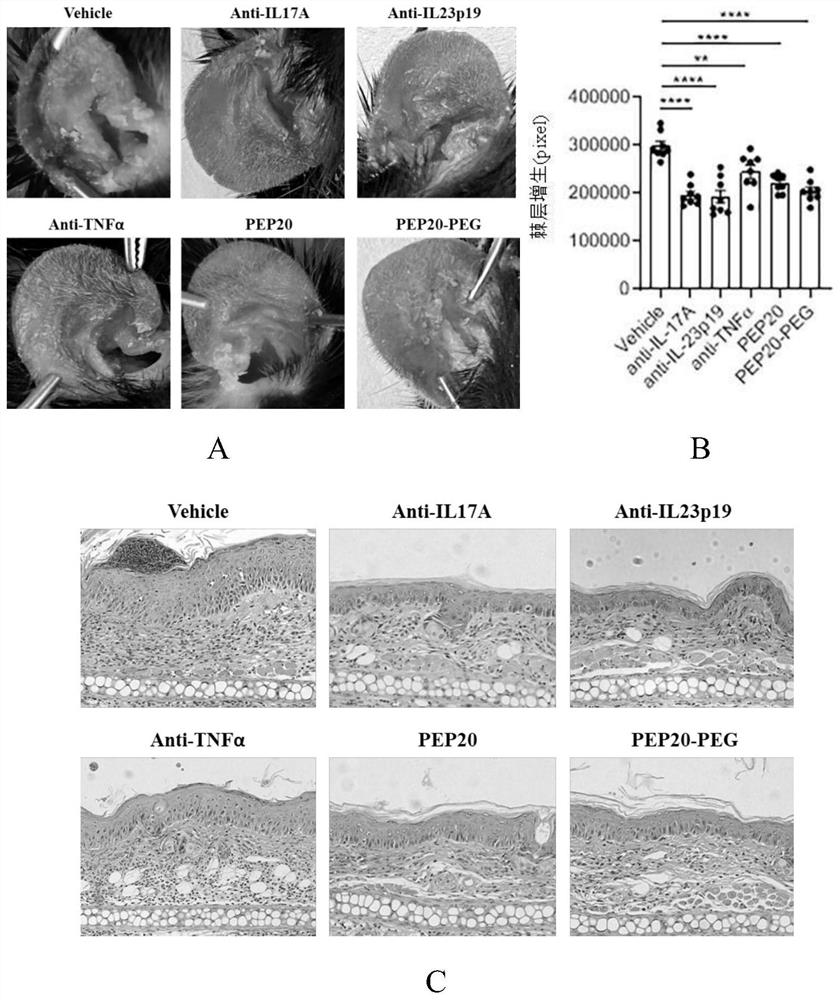
[0101] Example 3, PEP20 and PEP20-PEG can effectively alleviate the signs of psoriasis in mice induced by IMQ
[0102] SPF level mice were randomly divided into 6 groups, including Vehicle group (i.e. solvent group), 3 positive control groups, PEP20 group and PEP20-PEG group, and IMQ was used to smear the inside and outside of mouse ear skin, 30mg / mouse, total After smearing for 7 days, an IMQ-induced psoriasis model in mice was established, and solvent, ainti-IL17, ainti-IL23p19, ainti-TNFα, PEP20 and PEP20-PEG were respectively performed on the second day after IMQ administration (a small amount of dissolution first In HBSS, then dilute with normal saline to use the concentration of 80μg / 200μl) tail vein administration, the dose is 80μg / 20g mice, once a day, wherein antibody drugs ainti-IL17, ainti-IL23p19, ainti-TNFα are used as positive control drugs . Ear skin thickness was measured regularly every day from the day of induction. The mice were treated on the 7th day of I...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com