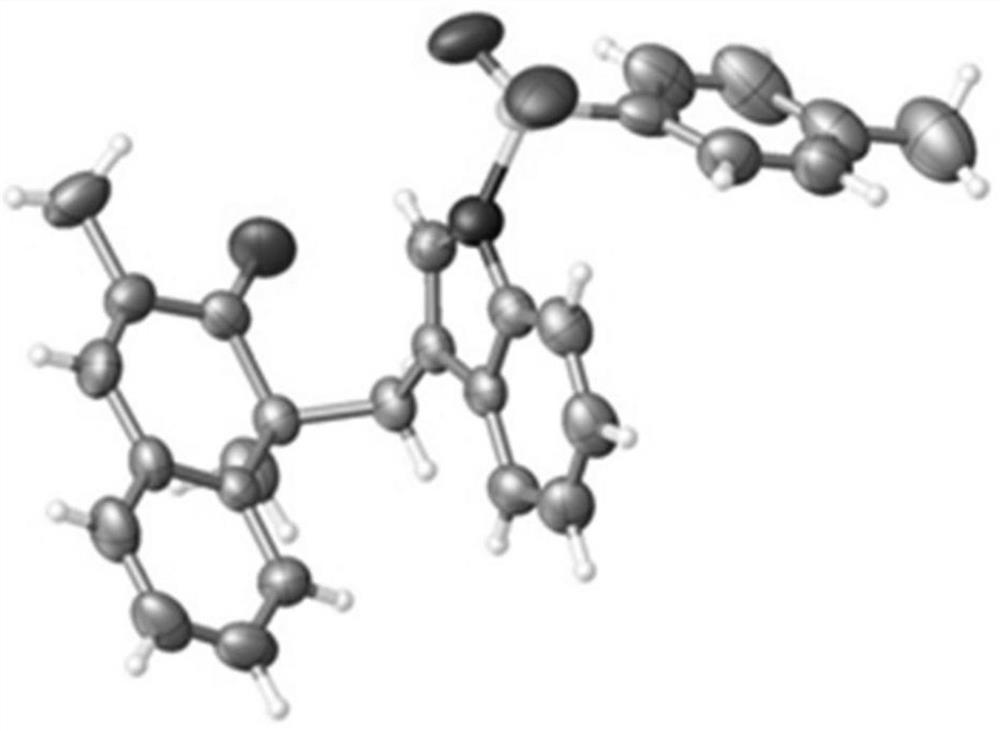
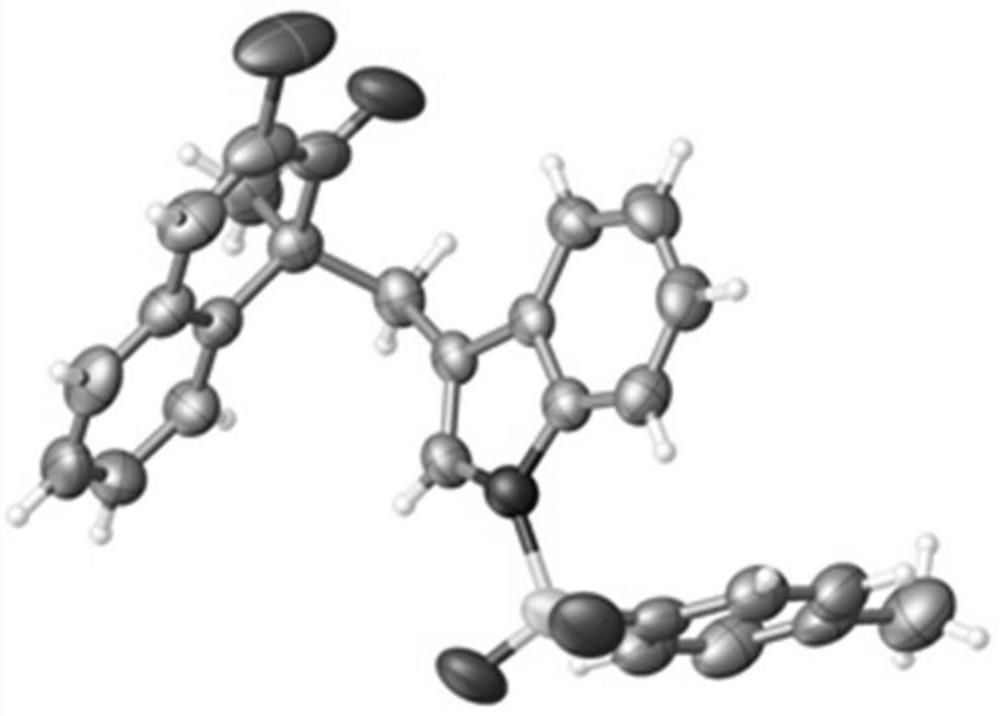
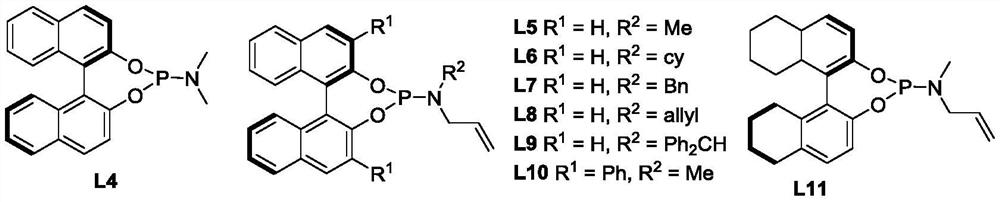
Method for synthesizing indole terpene analogue through Heck cascade reaction
A tandem reaction and indole terpene technology, applied in chemical instruments and methods, compounds of group 4/14 elements of the periodic table, organic chemistry, etc., to achieve high enantioselectivity of products
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Taking 1,3-dimethyl-2-naphthol 1a and N-2-iodophenyl-N-Ts-allenamine 2a to generate 3aa as an example, the reaction conditions are optimized, and the reaction equation is as follows:
[0033]
[0034]
[0035]
[0036] a Unless otherwise specified, the reaction steps are as follows: 1a (0.1mmol), 2a (0.12mmol), [Pd(C 3 h 5 )Cl] 2 (xmol%), ligand (y mol%), base (1.5equiv), 1.0mL solvent, in N 2 React under protection at T°C for 12 hours. b separation yield, c Determination by chiral high performance liquid chromatography. d 3aa yields and ee values were isolated after recrystallization.
[0037]During the screening of reaction conditions, the effect of the ligand on the reaction was first investigated (entries 1-5). At the same time, the effects of different solvents, reaction temperature (-20°C to +25°C), ligand L, palladium catalyst ratio and different bases (such as potassium carbonate, cesium carbonate, etc.) on the reaction were investigated, and L...
Embodiment 2
[0040]
[0041] Under nitrogen protection, in a Schlenk tube equipped with a magneton, 1-methyl-3-ethyl-β-naphthol (0.1 mmol), N-2-iodophenyl-N-Ts-allenamine (0.11 mmol), [Pd(C 3 h 5 )Cl] 2 (1mol%), L5 (1mol%) and Cs 2 CO 3 (0.15mmol), sealed with a rubber stopper. Syringe 1.0 mL of dry toluene into the reaction tube. The reaction tube was placed at 0°C and stirred overnight. The reaction solution was quickly passed through a short silica gel column, eluting with dichloromethane / methanol = 15 / 1, and the solution was concentrated in vacuo to obtain a crude product. Purification by silica gel column chromatography gave 3ba as a yellow solid with a yield of 91% and 93% ee. 1 H NMR (600MHz, CDCl 3 )δ7.81(d, J=7.8,1H),7.49-7.44(m,2H),7.41(d,J=7.2Hz,1H),7.34(td,J=7.2,1.2Hz,1H),7.29 (td,J=7.8,1.8Hz,1H),7.22(d,J=7.8Hz,1H),7.20-7.13(m,4H),7.12-7.07(m,1H),6.87(s,1H), 6.45(s,1H),3.50(d,J=14.3Hz,1H),3.07(d,J=14.3Hz,1H),2.34(s,3H),2.20-2.12(m,1H),2.10-2.02 (m,1H),1.59(s,3H),0...
Embodiment 3
[0043]
[0044] Under nitrogen protection, in a Schlenk tube equipped with a magneton, 1-methyl-3-allyl-β-naphthol (0.1 mmol), N-2-iodophenyl-N-Ts-allenamine ( 0.11mmol), [Pd(C 3 h 5 )Cl] 2 (1mol%), L5 (1mol%) and Cs 2 CO 3 (0.15mmol, 1.5 equivalents), sealed with a rubber stopper. Syringe 1.0 mL of dry toluene into the reaction tube. The reaction tube was placed at 0°C and stirred overnight. The reaction solution was quickly passed through a short silica gel column, eluting with dichloromethane / methanol = 15 / 1, and the solution was concentrated in vacuo to obtain a crude product. Then purified by silica gel column chromatography, the yellow oil 3ca was obtained with a yield of 91%, 92% ee. 1 H NMR (400MHz, CDCl 3 )δ7.82(d, J=8.0Hz, 1H), 7.51-7.45(m, 2H), 7.43(d, J=7.6Hz, 1H), 7.37(td, J=7.2, 1.2Hz, 1H), 7.30(td,J=7.6,1.6Hz,1H),7.25-7.17(m,2H),7.18-7.12(m,3H),7.10(td,J=7.6,1.1Hz,1H),6.82(s, 1H),6.43(s,1H),5.55-5.20(m,1H),4.95-4.72(m,2H),3.49(d,J=14.0Hz,1H),3.07(d,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com