Water-soluble cationic polyamidine and preparation method thereof
A technology of water-soluble cationic polyamidine, which is applied in the field of water-soluble cationic polyamidine and its preparation, can solve the problems of unfriendly environment and achieve the effects of environmental friendliness, simple operation and improved biocompatibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0033] The present invention provides a method for preparing water-soluble cationic polyproducts, including:
[0034] S1, in an organic solvent, dinitrobenzene and N, N-dibromobethalate, and a mid-secondary amine reaction to obtain a tert-butoxycarbonyl substituted polyhydration, the reaction formula is as follows:
[0035]
[0036] S2, remove the tert-butoxycarbonyl group to obtain a water-soluble cationic polyproduct.
[0037] Among them, R 1 R 2 Alkyl or aryl, R 1 R 2 Similarly or different, the specific structure of R1 and R2, this is not one by one, R 1 R 2 Depending on the specific structure of the mid-second amine monomer.
[0038] The reaction temperature was 40 ° C to 80 ° C, and the reaction time was 6 H-72H. The molar ratio of dinitrobenylbenzene and N, N-dibrominine and midupermide is 1: 2: 0.5-2, and the concentration of 0.01 to 10 mol / L of dinitrobenzene. The organic solvent is chloroform or methanol, but it is not limited thereto, and may be other organic solven...
Embodiment 1
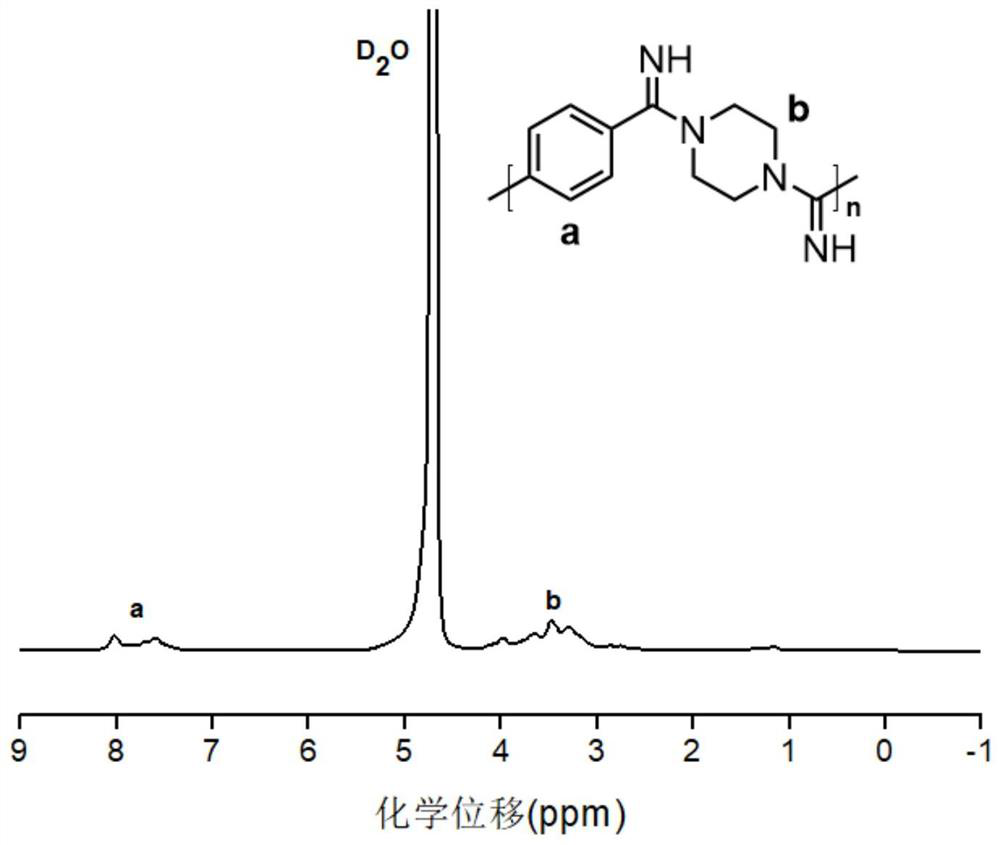
[0056] Double primary amine monomer selection piperazine: accurately weigh dinitrobenzene (22 mg, 0.1 mmol) and N, N-dibrominamate (54.6 mg, 0.2 mmol) in the reaction bottle, and accurate The addition of potassium phosphate (106.3 mg, 0.4 mmol) was added to the reaction flask, and then 0.5 mL-2 mL of chloroform was added to the reaction bottle and heated to 40 ° C to 70 ° C, and then mixed with piperazine (8.4 mg, 0.1 mmol), the maintenance temperature was 40 ° C to 70 ° C and stirred for 6-72 h, and the reaction was stopped. The crude product was dissolved in anhydrous methanol by sinking from an unhautus to anhydrous methanol, and the dialysis was dialyzed in anhydrous methanol (the dialysis bag was quantified by 1000 Da), and the tenthoacarbonyl substituted polymer was concentrated.
[0057] The above polymer (21 mg, 0.05 mmol) is accurately weighed in an appropriate amount of chloroform or dichloromethane, and the reaction bottle is placed in a low temperature reactor and the ...
Embodiment 2
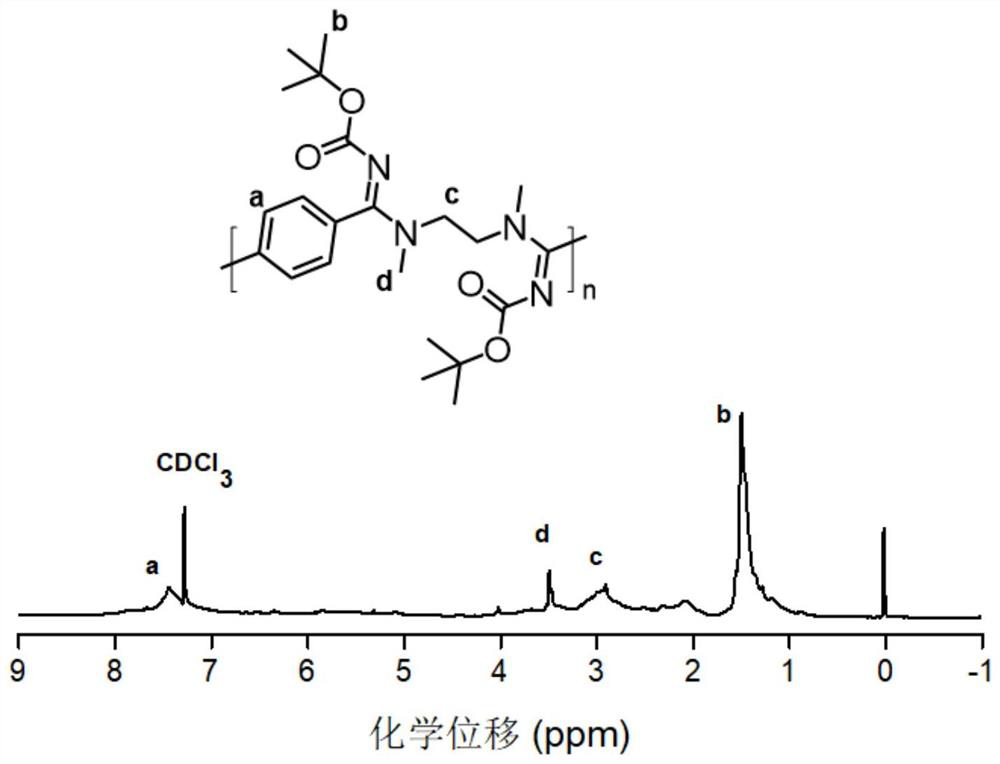
[0059] Double primary amine monomer selected n, N-dimethyl-1,3-ethylenediamine: accurate weight dinomide (22 mg, 0.1 mmol) and N, N-dibromoamate (54.6 Mg, 0.2 mmol) was placed in the reaction flask, accurately weighed potassium phosphate (106.3 mg, 0.4 mmol) to add the reaction bottle, then weighed 0.5 mL-2 mL of chloroform and heated to 40 ° C to 70 ° C, After stirring, N, N-dimethyl-1,3-ethylenediamine monomer (10.7 μL, 0.1 mmol) was added, and the maintenance temperature was 40 ° C to 70 ° C and stirred for 6-72 h, and the reaction was stopped. The crude product was dissolved in anhydrous methanol by sinking from an unhautus to anhydrous methanol, and the dialysis was dialyzed in anhydrous methanol (the dialysis bag was quantified by 1000 Da), and the tenthoacarbonyl substituted polymer was concentrated. The nuclear magnetomy of the obtained tert-butoxycarbonyl substituted polymer is figure 2 Indicated.
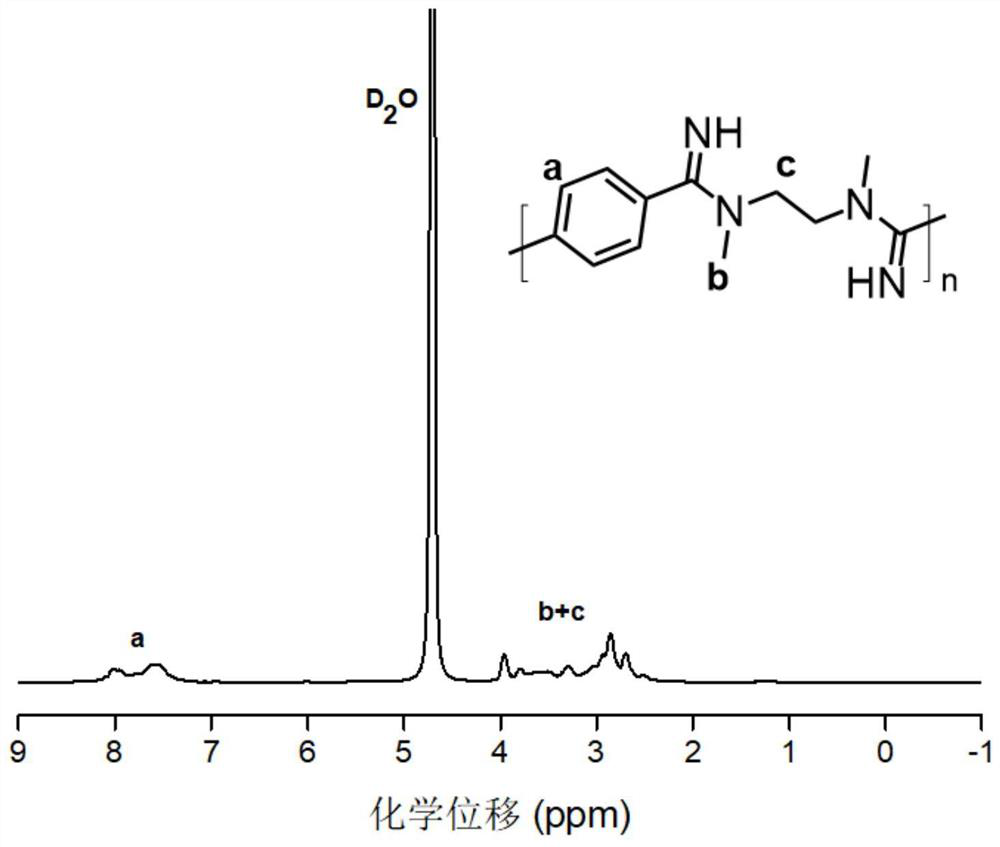
[0060] The above polymer (21.5 mg, 0.05 mmol) was accurately weighed in a...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Weight average molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com