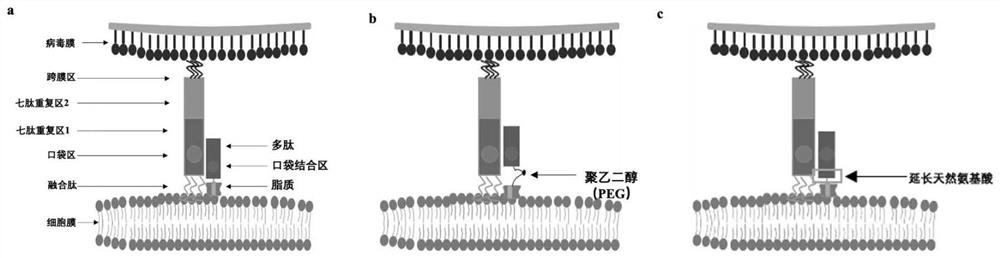
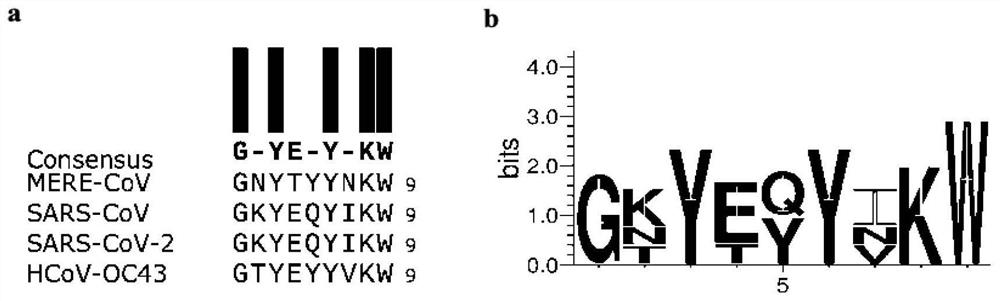
Polypeptide, preparation method and application thereof
A kind of use and activity-inhibiting technology, which is applied in the field of polypeptide drugs, can solve the problems of immune response and reduce the enzyme stability of polypeptide drugs, and achieve the effect of good stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0094] The invention also provides a preparation method of the polypeptide or fusion protein. The method includes introducing the expression vector into host cells, then culturing in a suitable medium, then harvesting or isolating the polypeptide or fusion protein in the supernatant, or in the case of intracellular expression, the host cells can be collected, and then the cells Cleavage is performed to collect these polypeptides or fusion proteins. In the case of a polypeptide or fusion protein, the polypeptide or fusion protein may be linked to a suitable signal peptide. The signal peptide is cleaved or not cleaved after harvest. These techniques are well known to those skilled in the art.
[0095] The polypeptides of the present invention can be prepared in the form of derivatives. For example, polypeptides can be palmitoylated or cholesterol modified. Methods for palmitoylation modification or cholesterol modification are well known. In one embodiment, palmitoylation o...
Embodiment 1
[0115]Embodiment 1: the preparation of SARS-CoV-2 (comprising its mutant), SARS-CoV, MERS-CoV, HCoV-OC43, HCoV-NL63, SARSr-CoV-Rs3367, SARSr-CoV-WIV1-CoV pseudovirus
[0116] experimental method:
[0117] 1. 24h before transfection, plate 293T cells in a 10cm tissue culture dish (2×106 / dish); culture with DMEM containing 10% FBS for 12h;
[0118] 2. Replace the cells with pre-warmed fresh DMEM medium (containing 10% FBS) 2 hours before transfection;
[0119] 3. Use two 1.5mL EP tubes for transfection, and add 500 μL of 0.9% NaCl solution to EP tube 1, which contains 20 μg of pcDNA3.1-SARS-2-S plasmid or pcDNA3.1-SARS- S plasmid or pcDNA3.1-MERS-S plasmid or pcDNA3.1-NL63-S plasmid or pcDNA3.1-OC43-S plasmid or pcDNA3.1-WIV1-S plasmid or pcDNA3.1-Rs3367-S plasmid or pcDNA3. 1-SARS-S mutant plasmid and pNL4-3.luc.RE plasmid. Related information can also be found in the literature under, S. # , Yan, L. # , Xu, W. # , Agrawal, A.S., Alsaissi, A., Tseng, C.T.K., Wang, Q., Du,...
Embodiment 2
[0125] Example 2: Inhibition of SARS-CoV-2 S protein-mediated cell-cell fusion experiment (cell-cellfusion)
[0126] experimental method:
[0127] 1. Transfect 293T cells with a plasmid encoding the S protein of SARS-CoV-2 (pAAV-IRES-GFP-SARS-CoV-2-S) and culture for 36-48h to obtain transfected cells as effector cells, Called 293T / 2019 / EGFP cells.
[0128] 2. Digest the 293T / 2019 / EGFP cells with 0.02% EDTA, centrifuge, resuspend the cells in fresh DMEM medium with 10% FBS, and adjust the cell concentration to 2×10 5 1 / mL, 50 μL was taken out and added to various test peptide drugs (50 μL) in serial dilution, and incubated at 37° C. for 30 min. Cells without drugs were used as positive control cells.
[0129] 3. Take 100 μL of the cell / drug mixture and add it to the target cell Huh-7 that has been plated on a 96-well plate. 5%CO 2 , Incubate at 37°C for 2-4 hours, observe and record the fusion of cells with the green fluorescent channel of a fluorescence microscope.
[0...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com