ANTIBODIES AGAINST SARS-CoV-1 OR SARS-CoV-2 AND USES THEREOF
A sars-cov-2 and antibody technology, applied in the direction of antibodies, medical preparations containing active ingredients, applications, etc., can solve problems such as limited clinical effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
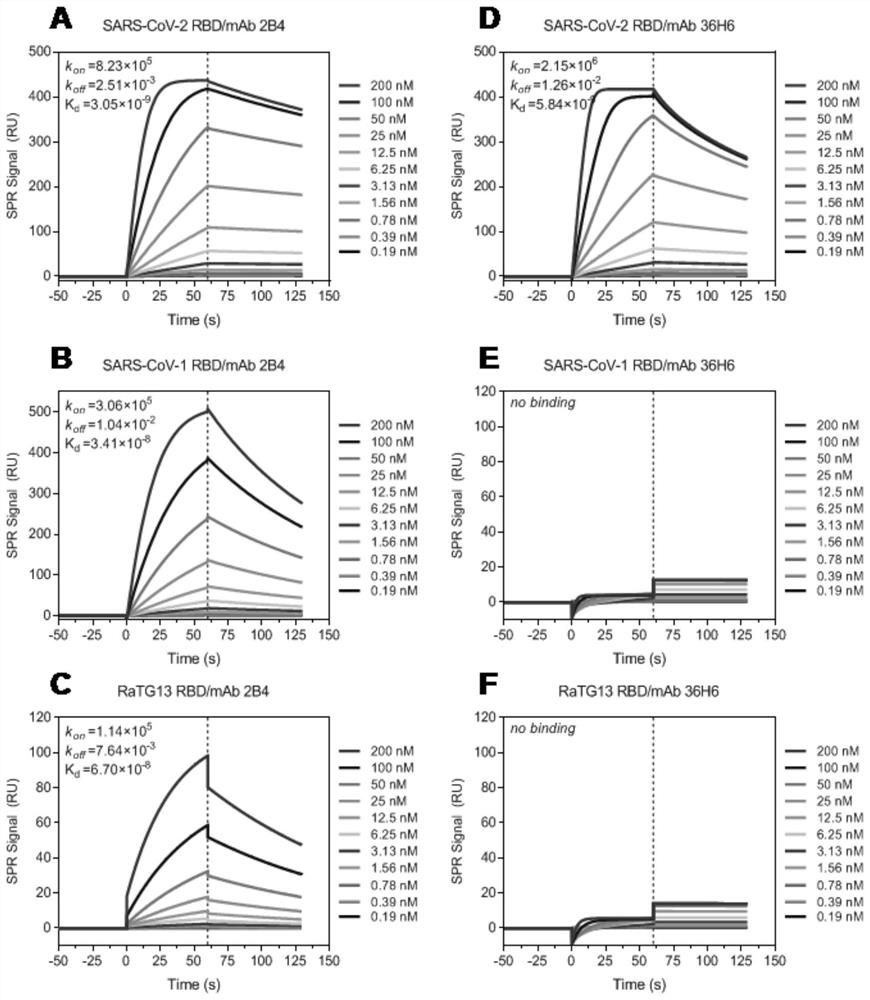
Image
Examples
Embodiment 1
[0242] Example 1: Synthesis of anti-SARS-CoV-2 receptor binding region RBD gene and construction of expression vector
[0243] Referring to the full gene sequence of SARS-CoV2-2 (MN908947.3), the nucleotide sequence encoding the SARS-CoV2-2 receptor binding domain RBD protein (corresponding to amino acids 316-550 of the S protein), according to the human code Sub-preferences are optimized. And, the signal peptide coding sequence (the guide sequence of human B2M) is connected to the N-terminal of the optimized nucleic acid sequence, and the polyhistidine polypeptide (6×His) that is convenient for affinity chromatography purification is connected to the C-terminal, and the protein Named as RBD-His, its nucleotide sequence is shown in SEQ ID NO:22, and its amino acid sequence is shown in SEQ ID NO:23. NEBuilder HiFi DNA AssemblyMaster Mix (NEB Company) was used to connect the above nucleotide sequence to the expression vector EIRBsMie-C18hA2dtSCT (enzyme cutting site is AgeI / Bgl...
Embodiment 2
[0244] Example 2: Expression and purification of the RBD antigen of SARS-CoV-2
[0245] 1. Expression of RBD antigen of SARS-CoV-2
[0246] with 3x10 6 The density of the ExpiCHO cells in the appropriate amount of medium ExpiCHO TM Expression Medium (Thermo Scientific Company) was cultured in Erlenmeyer shaker flasks at 37°C, 8% CO 2 , and cultured in a constant temperature shaker with an appropriate speed for 24 hours, until the cell density reached 6x10 6 Density.
[0247] Use ExpiFectamine according to the kit instructions TM The CHO Transfection Kit (ThermoScientific Company) transfected the vector EIRBsMie-RBDhis obtained in Example 1 into ExpiCHO cells. After continuing to culture under the same conditions for 17-24h, add the feed and enhancer provided in the kit, and replace the cells to 32°C, 5% CO 2 , and continue culturing for 6 days in a constant temperature shaker with an appropriate rotation speed.
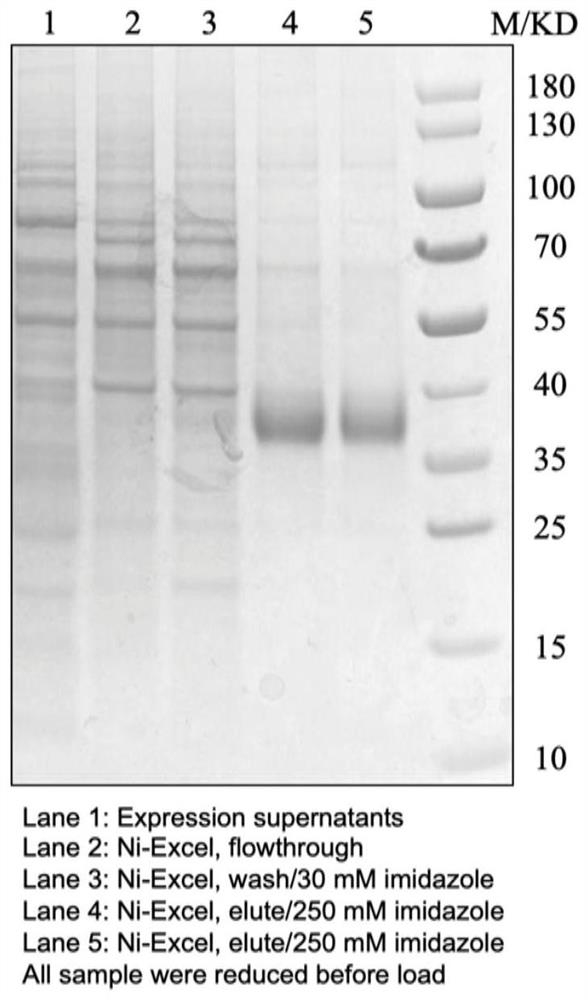
[0248] 2. Purification of the RBD antigen of SARS-CoV-2 ...
Embodiment 3
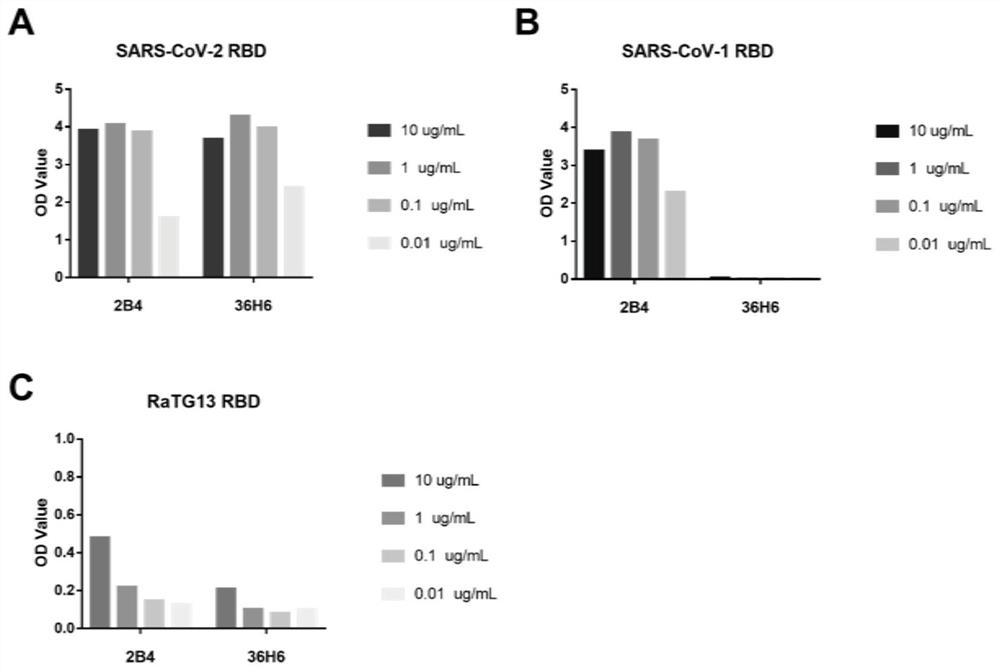
[0251] Example 3: Obtaining of mouse monoclonal antibody
[0252] 1. Mice Immunization
[0253] Standard in vivo immunization methods were used. For details, see Ed Harlow et al., "Antibodies A Laboratory Manual", Cold Spring Harbor Laboratory 1988. The brief procedure is as follows:
[0254] Use the SARS-CoV-2 RBD protein obtained in Example 2 to mix and emulsify in equal volumes with Freund's adjuvant. BALB / c female mice aged 6-8 weeks were immunized by multi-point subcutaneous injection in the bilateral groin, and a booster immunization was given every 2 weeks after the initial immunization. Serum antibody titers were measured by indirect ELISA, and fusion experiments were performed 4 weeks later.
[0255] The spleen was boosted 72 hours before the fusion of mouse spleen cells and mouse myeloma cells (SP2 / 0). The antigen for this immunization was the antigen without adjuvant, and the concentration was diluted to 1 mg / mL. Inject 50 μL of protein longitudinally along the s...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Equilibrium dissociation constant | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com