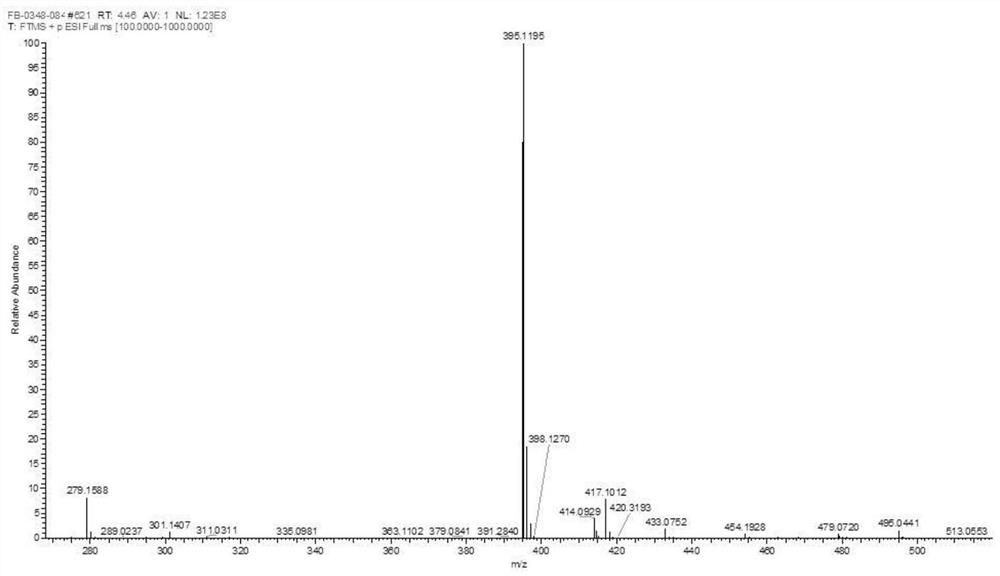
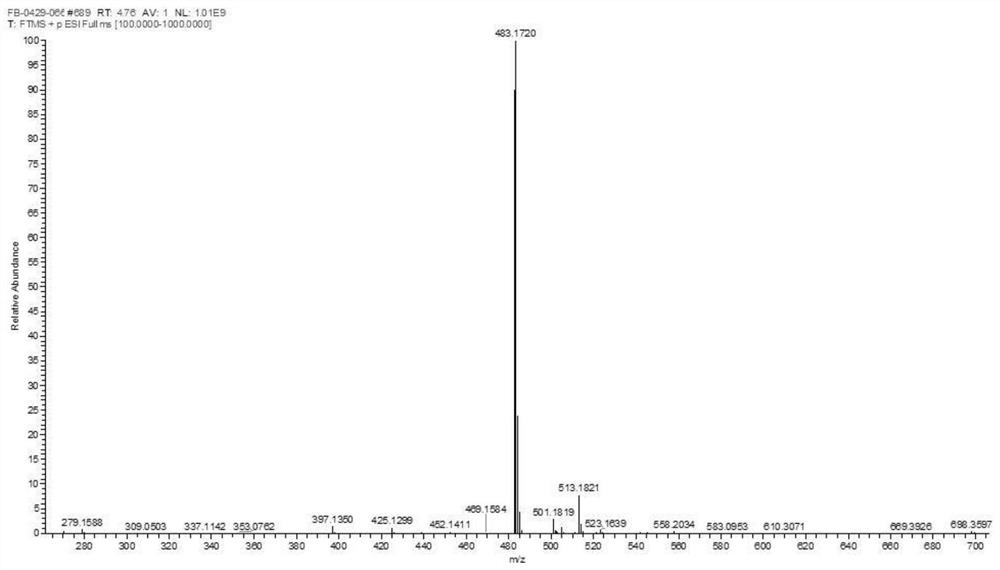
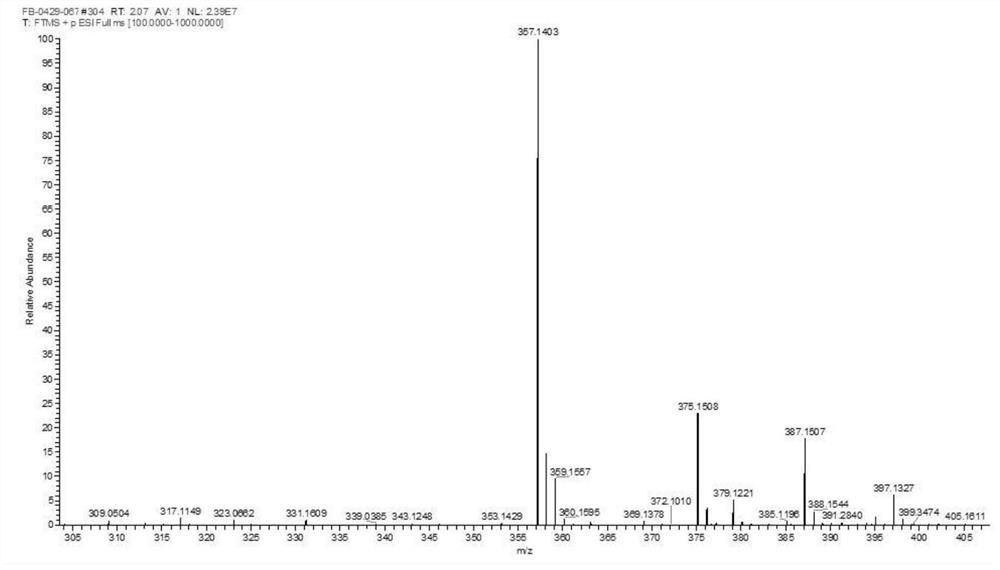
Method for synthesizing SAICAR
A technology of amino and imidazole, applied in the field of SAICAR synthesis, to achieve the effect of high yield, high purity of finished product and good stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0054] A synthetic (5-amino-1-((3aR,4R,6R,6aR)-3,4-dihydroxy-5-((phosphonooxy)methyl)tetrahydrofuran-2-yl)-1H-imidazole -4-carbonyl)-L-aspartic acid is prepared according to the following steps:
[0055] (1) (2R,3R,4R,5R)-2-(Acetoxymethyl)-5-(6-oxo-1,6-dihydro-9H-purin-9-yl)tetrahydrofuran-3, Preparation of 4-diacetate:
[0056] Add inosine (8.04g, 30.0mmol) and 4-(dimethylamino)pyridine (0.37g, 3.0mmol, 0.10eq) into 45mL of dry pyridine, and add acetic anhydride (30.60g, 300mmol, 10 equivalents), then the reaction mixture was stirred at 0°C for 1 hour, and then stirred at room temperature for 5 hours, and the reaction was monitored by LC-MS. After the reaction is complete, quench the reaction with 5 mL of cold methanol, concentrate the obtained crude product into a cold solution of ethyl acetate and n-hexane (20 mL) with a volume ratio of 1:3, stir evenly, filter, and filter the cake with a volume ratio of 1:3 ethyl acetate, n-hexane (5mL), ethyl acetate (5mL), and dried i...
Embodiment 2
[0077] A synthetic (5-amino-1-((3aR,4R,6R,6aR)-3,4-dihydroxy-5-((phosphonooxy)methyl)tetrahydrofuran-2-yl)-1H-imidazole -4-carbonyl)-L-aspartic acid is prepared according to the following steps:
[0078](1) (2R,3R,4R,5R)-2-(Acetoxymethyl)-5-(6-oxo-1,6-dihydro-9H-purin-9-yl)tetrahydrofuran-3, Preparation of 4-diacetate: Add inosine (8.04g, 30.0mmol) and 4-(dimethylamino)pyridine (0.37g, 3.0mmol, 0.10eq) into 45mL of dry pyridine, dropwise at 0°C Acetic anhydride (30.60 g, 300 mmol, 10 equiv) was added, then the reaction mixture was stirred at 0° C. for 1 hour, warmed to room temperature and stirred for 5 hours, and the reaction was monitored by LC-MS. After the reaction is complete, quench the reaction with 5 mL of cold methanol, concentrate the obtained crude product into a cold solution of ethyl acetate and n-hexane (20 mL) with a volume ratio of 1:3, stir evenly, filter, and filter the cake with a volume ratio of 1:3 ethyl acetate, n-hexane (5mL), ethyl acetate (5mL), and ...
Embodiment 3
[0099] A synthetic (5-amino-1-((3aR,4R,6R,6aR)-3,4-dihydroxy-5-((phosphonooxy)methyl)tetrahydrofuran-2-yl)-1H-imidazole -4-carbonyl)-L-aspartic acid is prepared according to the following steps:
[0100] (1) (2R,3R,4R,5R)-2-(Acetoxymethyl)-5-(6-oxo-1,6-dihydro-9H-purin-9-yl)tetrahydrofuran-3, Preparation of 4-diacetate: Add inosine (8.04g, 30.0mmol) and 4-(dimethylamino)pyridine (0.37g, 3.0mmol, 0.10eq) into 45mL of dry pyridine, dropwise at 0°C Acetic anhydride (30.60 g, 300 mmol, 10 equiv) was added, then the reaction mixture was stirred at 0° C. for 1 hour, warmed to room temperature and stirred for 5 hours, and the reaction was monitored by LC-MS. After the reaction is complete, quench the reaction with 5 mL of cold methanol, concentrate the obtained crude product into a cold solution of ethyl acetate and n-hexane (20 mL) with a volume ratio of 1:3, stir evenly, filter, and filter the cake with a volume ratio of 1:3 ethyl acetate, n-hexane (5mL), ethyl acetate (5mL), and...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com