Powdered polycarbodiimide compound and ester resin composition
A polycarbodiimide and ester resin technology, applied in the field of ester resin compositions, can solve problems such as tackifying, terminal isocyanate group residue, etc., and achieve the effects of improving hydrolysis resistance, excellent operability, and excellent handling properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0112] [Preparation method of powder polycarbodiimide compound]
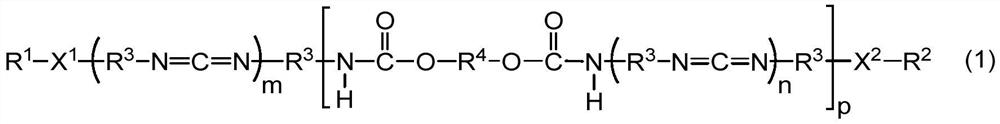
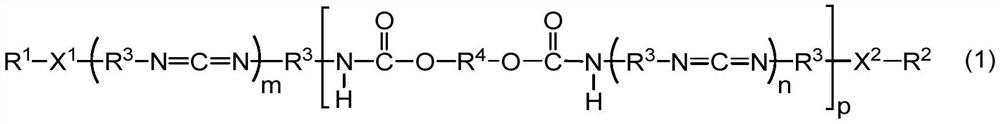
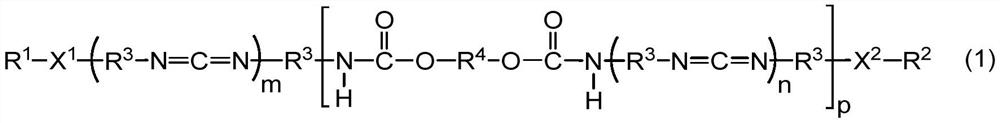
[0113] The method for preparing the powdery polycarbodiimide compound is not particularly limited, and known methods can be used. For example, the powdery polycarbodiimide compound can be obtained by pulverizing a solid carbodiimide compound obtained by the synthesis methods shown in (1) to (3) below.
[0114] (1) Carbodiimidization of the diisocyanate compound in the presence of a catalyst to obtain isocyanate-terminated polycarbodiimide, followed by adding the organic compound (blocking agent) and the diol compound, Process for Carbamate Reaction and Capping Reaction
[0115] (2) Mix the diisocyanate compound, the diol compound and the organic compound (capping agent), and perform urethanization reaction, carbodiimidization reaction and capping reaction in the presence of a catalyst Methods
[0116] (3) After urethanizing a part of the diisocyanate compound with the diol compound to obtain an isocyanate-ter...
Embodiment 1
[0173] Put 100 parts by mass of HMDI and 0.5 parts by mass of 3-methyl-1-phenyl-2-phosphole-1-oxide as a carbodiimidation catalyst in a reaction with a reflux tube and a stirrer In the container, stirring and mixing were carried out at 185° C. for 24 hours under nitrogen flow to perform carbodiimidization reaction to obtain isocyanate-terminated polycarbodiimide.
[0174] IR spectrum measurement of the obtained isocyanate-terminated polycarbodiimide confirmed that the wavelength was 2150 cm -1 before and after the absorption peaks of the carbodiimide group. In addition, the amount of terminal NCO was 8.19% by mass, and the degree of polymerization of the carbodiimide group obtained by the above measurement method was 3.5.
[0175] Next, 8.8 parts by mass of MPG and 4.0 parts by mass of EG were added to the isocyanate-terminated polycarbodiimide at 150° C. under a nitrogen stream, heated to 180° C., stirred and mixed for 2 hours, and reacted.
[0176] Measured by IR spectrum,...
Embodiment 2-6 and comparative example 1-8
[0178] Except that the diisocyanate compound, organic compound (blocking agent) and diol compound in Example 1 were changed to the compounds shown in the following Table 1, the same operation was carried out as in Example 1 to synthesize each powder polycarbonate imine compounds.
[0179] In addition, in Comparative Examples 7 and 8, the obtained polycarbodiimide compound was not a solid but a viscous liquid, and a powder polycarbodiimide compound could not be obtained.
[0180] [Measurement and Evaluation of Powdered Polycarbodiimide Compounds]
[0181] The measurement and evaluation of the items shown below were performed on each powder polycarbodiimide compound obtained in the above-mentioned Examples and Comparative Examples. These measurement and evaluation results are summarized in Table 1 below.
[0182] In addition, since the polycarbodiimide compounds obtained in Comparative Examples 7 and 8 were not powders, melting point measurement and blocking evaluation were no...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com