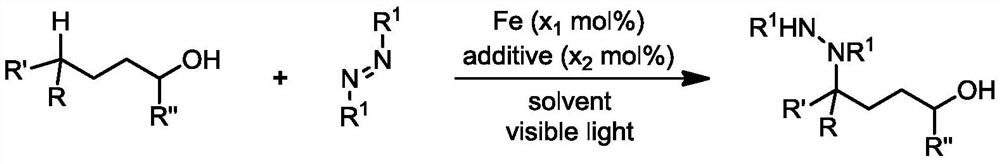
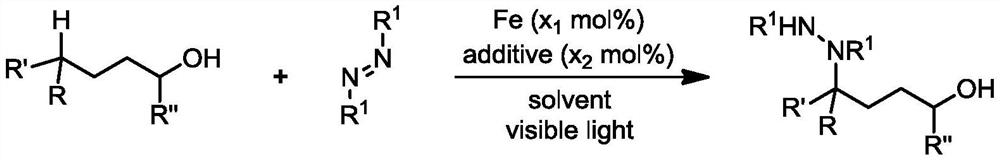
Preparation method of 4-aminated alcohol through iron catalysis
A technology for amination of alcohols and iron compounds, which can be applied in chemical instruments and methods, organic chemistry, compounds of elements of Group 4/14 of the periodic table, etc. The problem of high price, to achieve the effect of short reaction time, mild conditions and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Add iron trichloride (0.004 mmol), tetrabutylammonium chloride (0.004 mmol), tert-butoxycarbonyl substituted azo compound (0.4 mmol), phenylbutanol (1.2 mmol) to the dry reaction tube successively. mol), anhydrous acetonitrile (4 milliliters), after the addition, stir to dissolve, mix evenly, place the reaction tube under the light (hv) with a wavelength of 390nm and keep stirring. Next, the reaction mixture was transferred to a flask, and the crude product was obtained by distillation under reduced pressure. Flash column chromatography obtained 127.0 mg of the aminated product of phenylbutanol, with a yield of 82%. The product was a colorless oily substance. 1H NMR (400MHz, CDCl3) δ7.46–7.18(m,5H,Ar-H),6.72–5.73(m,1H,NH),5.59–5.00(m,1H,CH),3.87–3.50(m, 2H,CH2),2.39(brs,1H,OH),2.24–1.59(m,4H,CH2×2),1.59–0.95(m,18H,Boc×2).13CNMR(101MHz,CDCl3)δ156.0,155.1, 139.7, 128.4, 127.9, 127.6, 81.4, 81.1, 62.2, 59.2, 29.6, 28.2, 27.4. HRMS (ESI+) m / z: Calcd for C21H34N2O6Na+[M+Na...
Embodiment 2
[0038]According to the method described in Example 1, the difference is that the amount of reagent used is: iron trichloride (0.008 mmol), tetrabutylammonium chloride (0.004 mmol), tert-butoxycarbonyl substituted azo compound (0.4 mmol) mol), 4-methoxyphenylbutanol (208 microliters, 216.7 mg, 1.20 mmol), anhydrous acetonitrile (4 milliliters); the reaction tube was irradiated under light (hv) with a wavelength of 390nm and heated to 60 ℃ and kept stirring, after the reaction was completed, the reaction tube was removed from the light source, the reaction mixture was transferred to a flask, and the crude product was obtained by distillation under reduced pressure, and the amination product of 4-methoxyphenylbutanol was obtained by flash column chromatography, 117.0 mg, yield 71%. 1HNMR (400MHz, CDCl3) δ7.34–7.14 (m, 2H, Ar-H), 6.83 (d, J=8.4Hz, 2H, Ar-H), 6.59–5.62 (m, 1H, NH), 5.60– 4.94(m,1H,CH),3.77(s,3H,CH3),3.78–3.53(m,2H,CH2),2.33–1.77(m,5H,OH+CH2×2),1.64–0.96(m, Boc×2)...
Embodiment 3
[0040] According to the method described in Example 1, the difference is that the amount of reagent used is: iron tribromide (0.012 mmol), tetrabutylammonium chloride (0.004 mmol), tert-butoxycarbonyl substituted azo compound (0.4 mmol) mol), 4-chlorophenylbutanol (240.8 mg, 1.2 mmol), anhydrous acetonitrile (4 milliliters); the reaction tube is placed in the light (hv) of wavelength 390nm to irradiate and heated to 80 ° C, stirring constantly, to be reacted After the completion, the reaction tube was removed from the light source, the reaction mixture was transferred to a flask, and the crude product was obtained by distillation under reduced pressure. The amination product of 4-chlorophenylbutanol was obtained by flash column chromatography, 127.5 mg, with a yield of 76%. 1H NMR (400MHz, CDCl3) δ7.36–7.21(m,4H,Ar-H),6.91–5.74(m,1H,NH),5.76–4.99(m,1H,CH),3.79–3.55(m, 2H,CH2),2.46(brs,1H,OH),2.22–0.99(m,22H,Boc×2+CH2×2).13C NMR(101MHz,CDCl3)δ156.1,155.0,138.1,133.4,130.1,129.3...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com