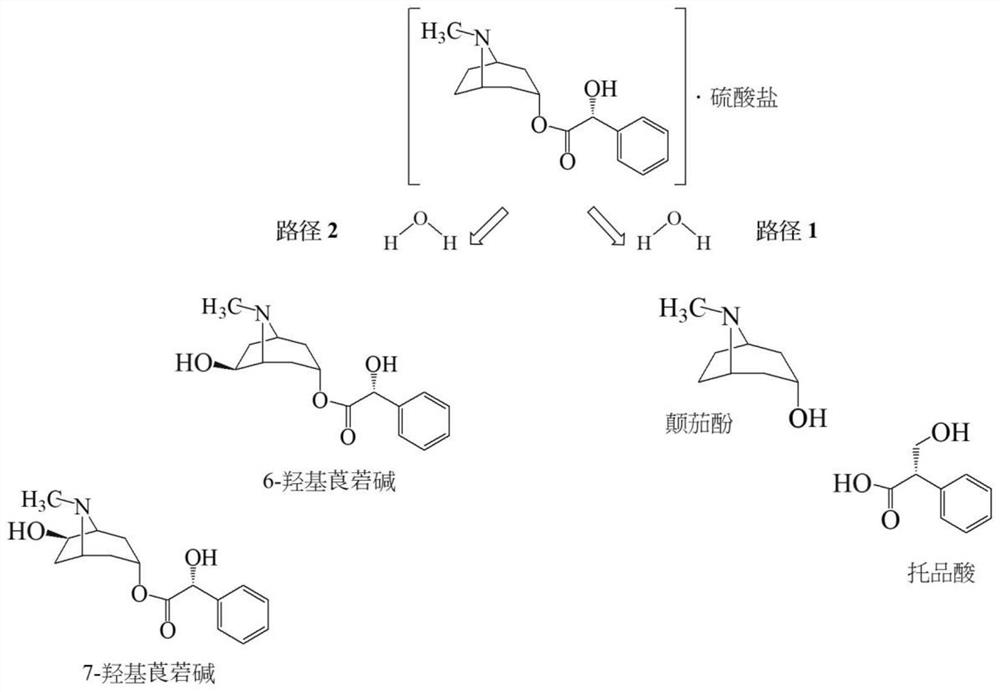
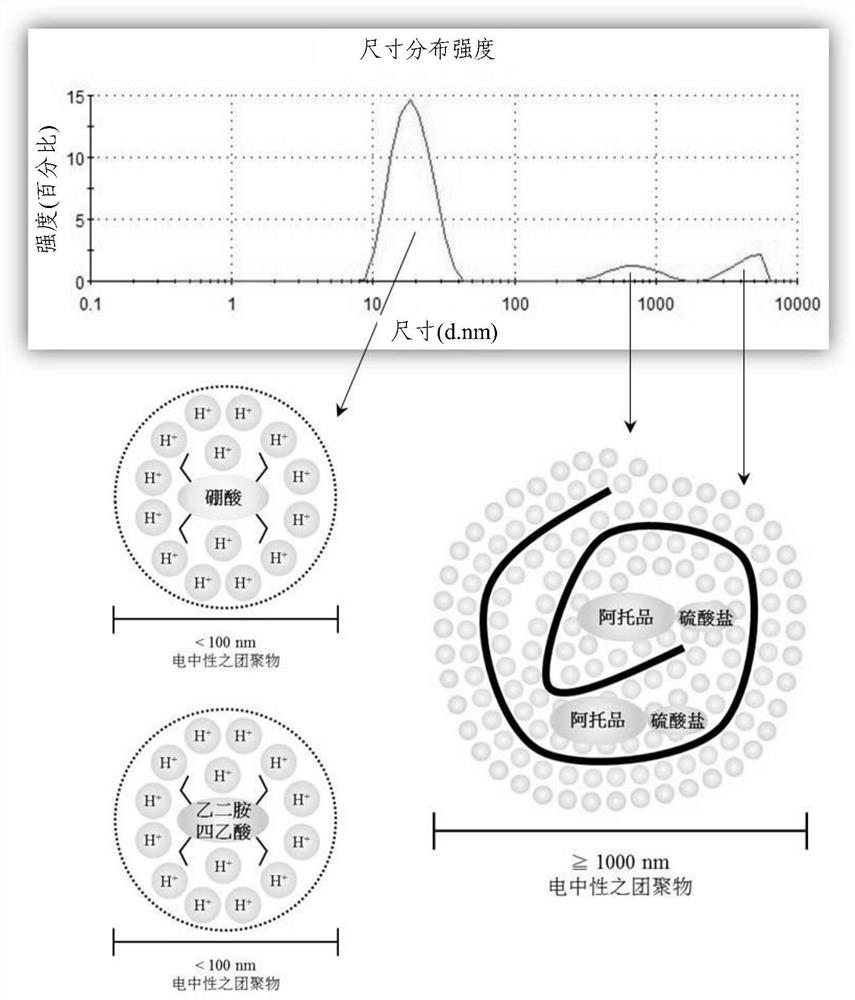
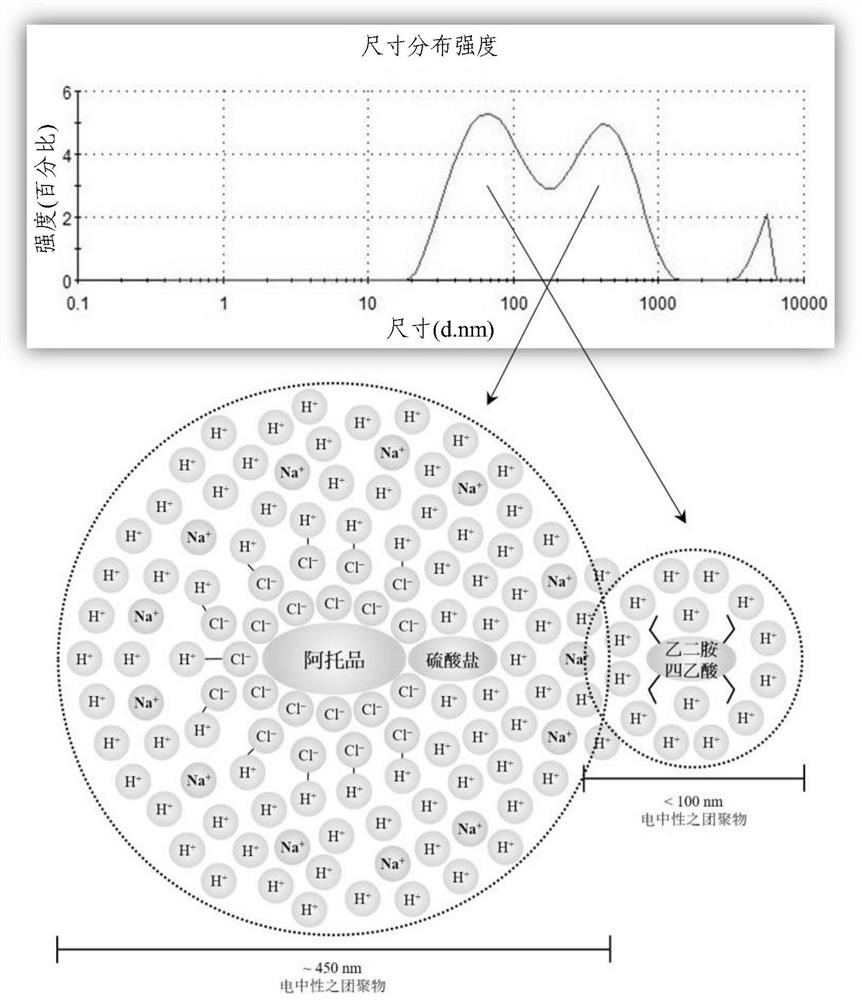
Ophthalmic pharmaceutical composition
A composition and drug technology, applied in the direction of drug combination, drug delivery, active ingredients of heterocyclic compounds, etc., can solve the problems of corneal epithelial cell damage, preservative eye allergy or irritation, and bacterial growth, etc., to improve related Substance problems, the effect of improving corneal penetration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-15
[0058] Embodiments 1-15 of the present invention provide an ophthalmic pharmaceutical composition, the formulation of which comprises: atropine sulfate, citric acid as a pH regulator, sodium chloride as an osmotic pressure regulator, and water; and the ophthalmic The pharmaceutical composition contains no preservatives. The content of each component of the ophthalmic pharmaceutical composition and its pH value and osmotic pressure parameters are shown in Table 1 below.
[0059] Table 1
[0060]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com