Cantharidin cream and preparation method thereof
A cantharidin and cream technology, which is applied in the directions of ointment delivery, pharmaceutical formulation, emulsion delivery, etc., can solve problems such as thinning and demulsification, and achieve the effects of improving stability and simplifying the preparation process.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Embodiment 1: the extraction of cantharidin
[0030] ① Pulverization: Pulverize the mylabris into a coarse powder with a pulverizer for later use.
[0031] 2. Cold soaking: add 2 times the amount of acetone (weight / volume ratio) of the weight of the cantharidin coarse powder, seal the container at room temperature, and leave it at rest for 72 hours each time, soaking three times in total;
[0032] ③ Filtration and distillation: filter the soaking liquid collected three times, collect the filter residue and send it to the dangerous goods warehouse for storage and centralized treatment. The filtrate is distilled under reduced pressure, and the temperature is controlled at 50-60°C. After recovering acetone, the residue is the mixture of cantharidin and cantharidin oil ;
[0033] ④Extraction and separation: add petroleum ether (60-90) to the mixture for extraction and separation (add petroleum ether in three times the amount of residue ), stir well and stand still for 15 m...
Embodiment 2
[0035] Embodiment 2: preparation cantharidin cream
[0036] 1. Formula
[0037] Table 1
[0038] name prescription dosage Dosage (g) Features Poloxamer P407 20% 200 matrix Poloxamer P188 5% 50 matrix ethanol 30% 300 solvent Cantharidin 0.025% 0.25 active ingredient purified water 44.975% 449.75 solvent Total 100% 1000 /
[0039] 2. Preparation method
[0040] (1) Weighed 200g of poloxamer P407 prescription and 50g of P188 prescription, added 449.75g of purified water of the prescription and stirred evenly, then put it in a refrigerator at 4°C and refrigerated overnight for use;
[0041] (2) Take by weighing 300g of ethanol and 0.25g of cantharidin in the prescription quantity, both are placed in the container, after dissolving and clarifying at 50°C, cool to 4°C, and stand-by;
[0042] (3) Take the mixed solution in (2) above, and slowly add the mixed solution in (1) above under stirring at 4°C (500rmp)...
Embodiment 3
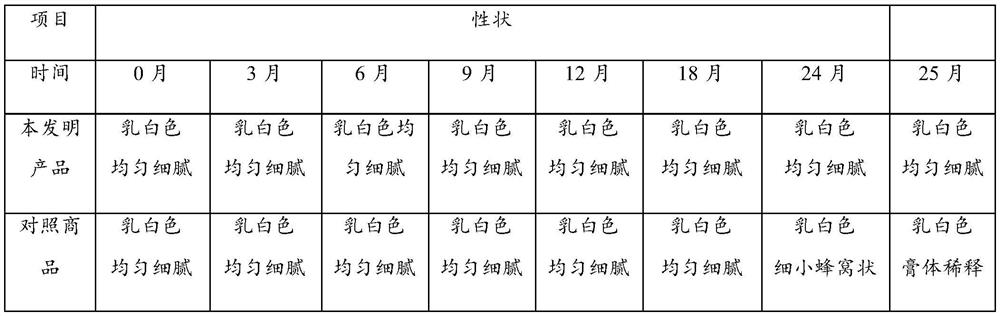
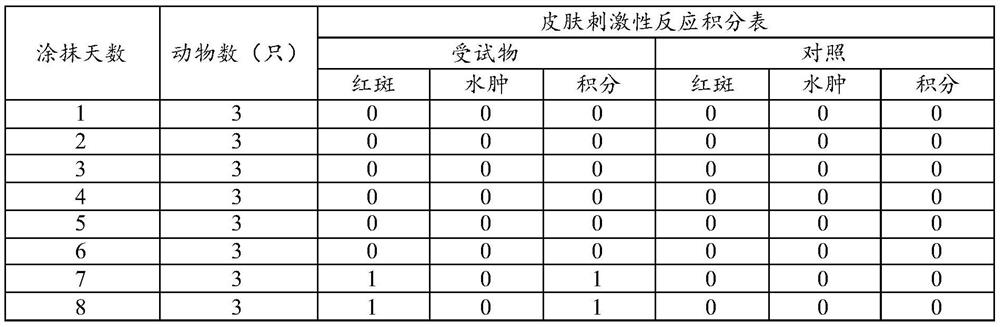
[0044] Embodiment 3: paste stability comparison
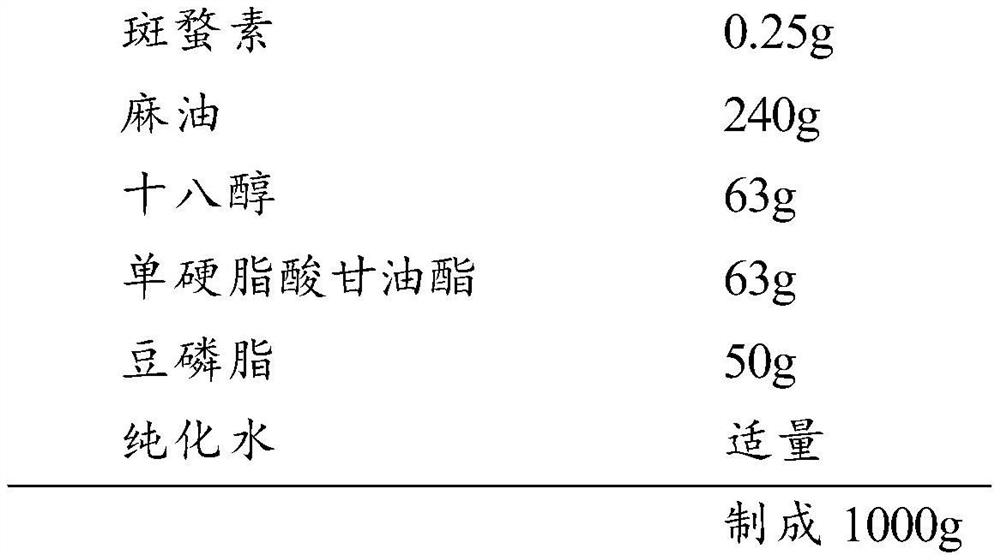
[0045] 1. Comparing the product formula
[0046] Formula (based on 1000g):
[0047]
[0048] 2. Comparison of product preparation process
[0049] Dissolve cantharidin in sesame oil to make cantharidin sesame oil solution (about 120°C); dissolve stearyl alcohol and glyceryl monostearate in cantharidin sesame oil solution; filter with 200 mesh sieve, 80°C-82°C Insulation standby;
[0050] (1) Mix the soybean lecithin and purified water of the prescribed amount to make a uniform soybean lecithin dispersion, heat up to 85°C, and keep it warm at 80°C to 85°C for later use;
[0051] (2) After checking that everything is normal in the paste making machine, turn on the power according to the SOP of the YGP250 vacuum paste making machine. After sealing the equipment container, turn on the cooling water to vacuum up to the highest vacuum degree;
[0052](3) Inhale the oil phase material into the container, and start the scraper ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com