A kind of polyquinoline dione compound and its preparation method and application
A technology of polyquinoline diones and compounds, which is applied in the fields of polymer chemistry and materials science, can solve the problems of seldom research on the construction of heterocyclic polymers, achieve high thermal stability and refractive index, wide source, and good functional group tolerance sexual effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
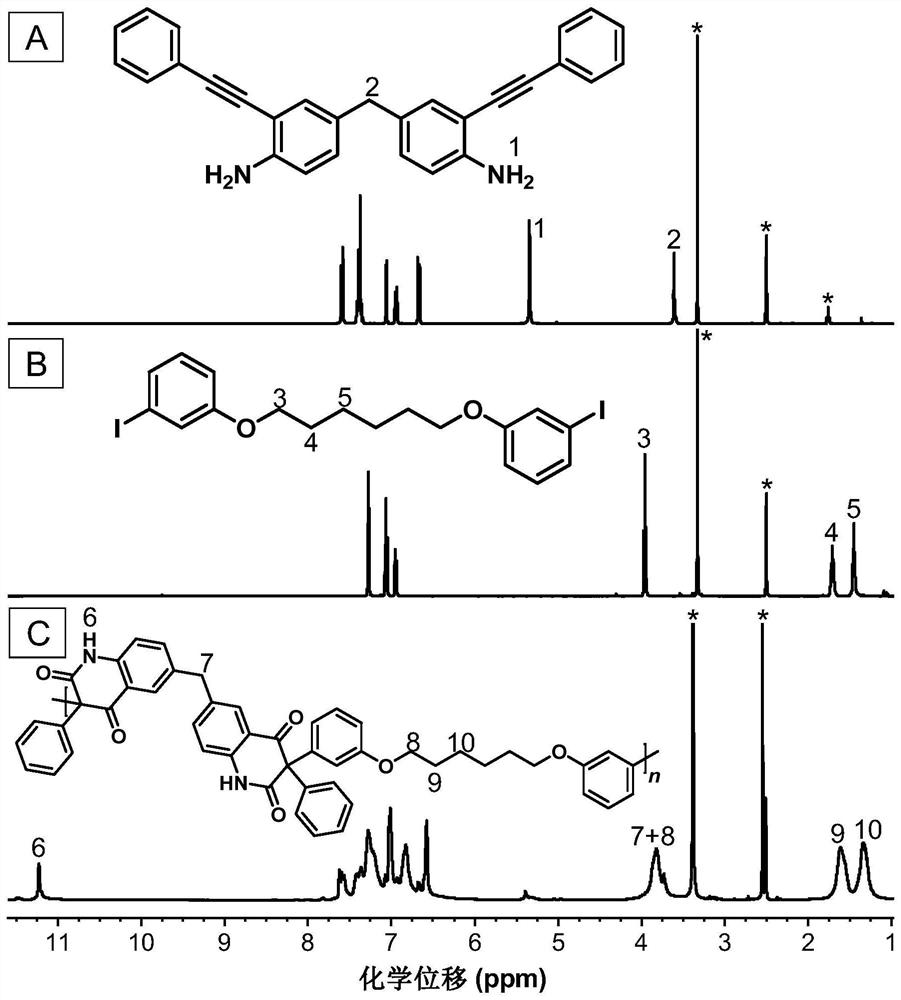
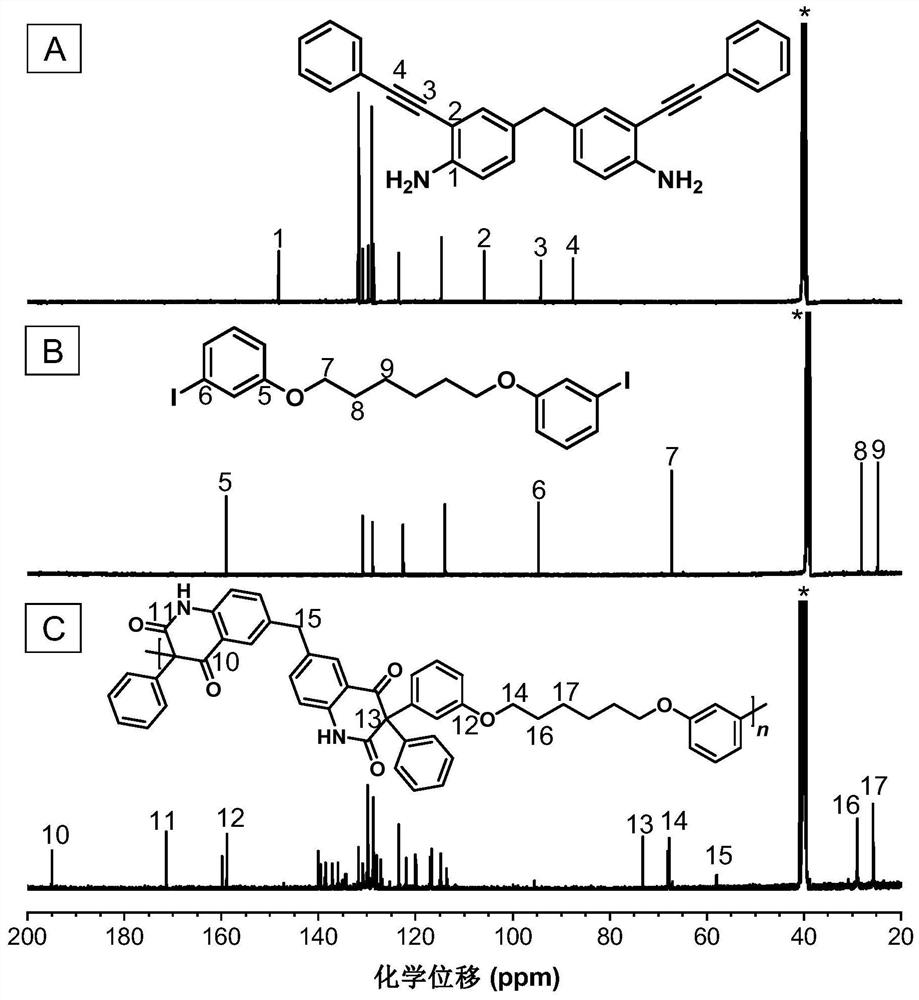
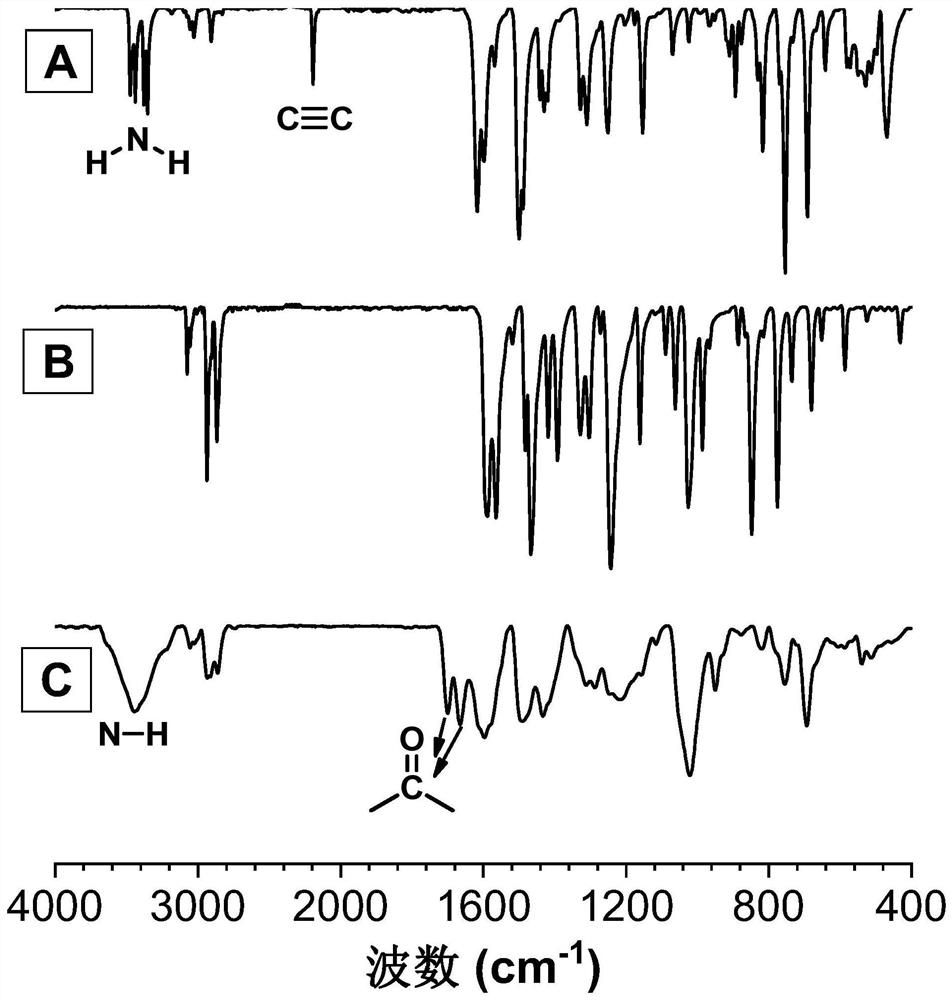
[0048] A kind of polyquinoline dione compound, its structural formula is shown as P1:
[0049]
[0050] Said polyquinoline dione compounds pass through CO 2, the polymerization reaction of bifunctional o-alkynyl aniline monomer and bifunctional aryl iodide monomer is prepared, and the reaction equation is as formula (1):
[0051]
[0052] Wherein, the o-iodoaniline intermediate can be synthesized with reference to the preparation method of the literature (Macromolecules, 2021,54(9): 4112-4119), and then it and phenylacetylene are synthesized by Sonogashira reaction to synthesize monomer M1; monomer N1 can refer to literature ( Chem. Commun., 2019, 55(47): 6755-6758). Synthesized.
[0053] The preparation steps of the described polyquinoline dione compounds are as follows:
[0054] To a 10 mL dry polymerization tube equipped with a magnetic stirrer was added M1 (0.1 mmol, 39.9 mg), N1 (0.1 mmol, 52.2 mg), bistriphenylphosphonium palladium dichloride (Pd (PPh) 3 ) 2 Cl...
Embodiment 2
[0057] A kind of polyquinoline dione compound, its structural formula is shown as P2:
[0058]
[0059] Said polyquinoline dione compounds pass through CO 2 , the polymerization reaction of bifunctional o-alkynyl aniline monomer and bifunctional aryl iodide monomer is prepared, and the reaction equation is such as formula (two):
[0060]
[0061]
[0062] Wherein, the synthesis method of the monomer M1 is the same as that in Example 1; the monomer N2 is purchased from Matrix Scientific Company.
[0063] The preparation steps of the described polyquinoline dione compounds are as follows:
[0064] M1 (0.1 mmol, 39.9 mg), N2 (0.08 mmol, 33.8 mg), Pd (PPh) were added to a 10 mL dry polymerization tube equipped with a magnetic stir bar 3 ) 2 Cl 2 (0.02mmol, 14mg) and Cs 2 CO 3 (0.2mmol, 65.3mg), with CO 2 three times, in CO 2 Under atmosphere, inject 0.33 mL of ultra-dry DMSO through a syringe. The reaction system was reacted at 80°C for 1 hour. After the reactio...
Embodiment 3
[0067] A kind of polyquinoline dione compound, its structural formula is shown as P3:
[0068]
[0069] Said polyquinoline dione compounds pass through CO 2 , the polymerization reaction of bifunctional o-alkynyl aniline monomer and bifunctional aryl iodide monomer is prepared, and the reaction equation is such as formula (three):
[0070]
[0071] Wherein, the synthesis method of the monomer M1 is the same as that in Example 1; the monomer N3 is purchased from Shanghai Bide Pharmaceutical Technology Co., Ltd.
[0072] The preparation steps of the described polyquinoline dione compounds are as follows:
[0073] M1 (0.1 mmol, 39.9 mg), N3 (0.12 mmol, 52.1 mg), Pd (PPh) were added to a 10 mL dry polymerization tube equipped with a magnetic stir bar 3 ) 2 Cl 2 (0.02mmol, 14mg) and Cs 2 CO 3 (0.2mmol, 65.3mg), with CO 2 three times, in CO 2 Under atmosphere, inject 2 mL of ultra-dry DMSO through a syringe. The reaction system was reacted at 140°C for 8 hours. After...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com