Polyquinazoline compound as well as preparation method and application thereof
A polyquinazoline and compound technology, applied in the fields of polymer chemistry and materials science, can solve the problems of cumbersome steps and low yield, and achieve the effects of simple process, wide source and excellent solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
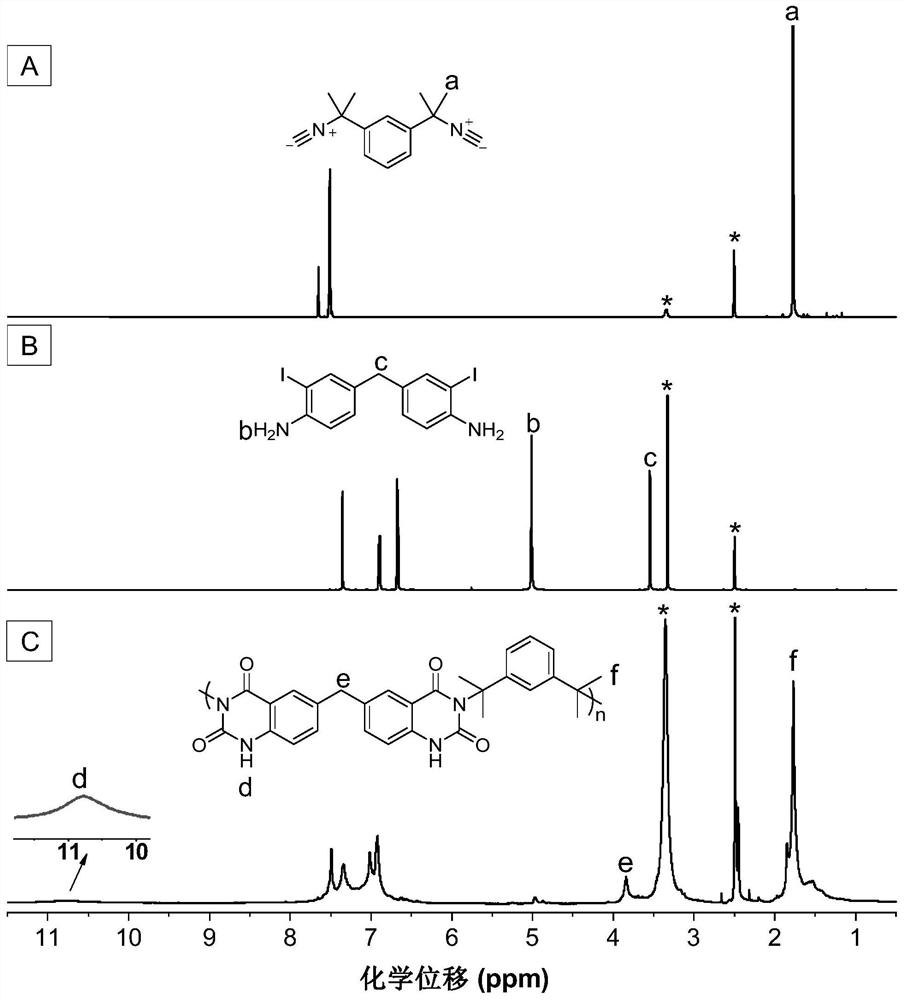
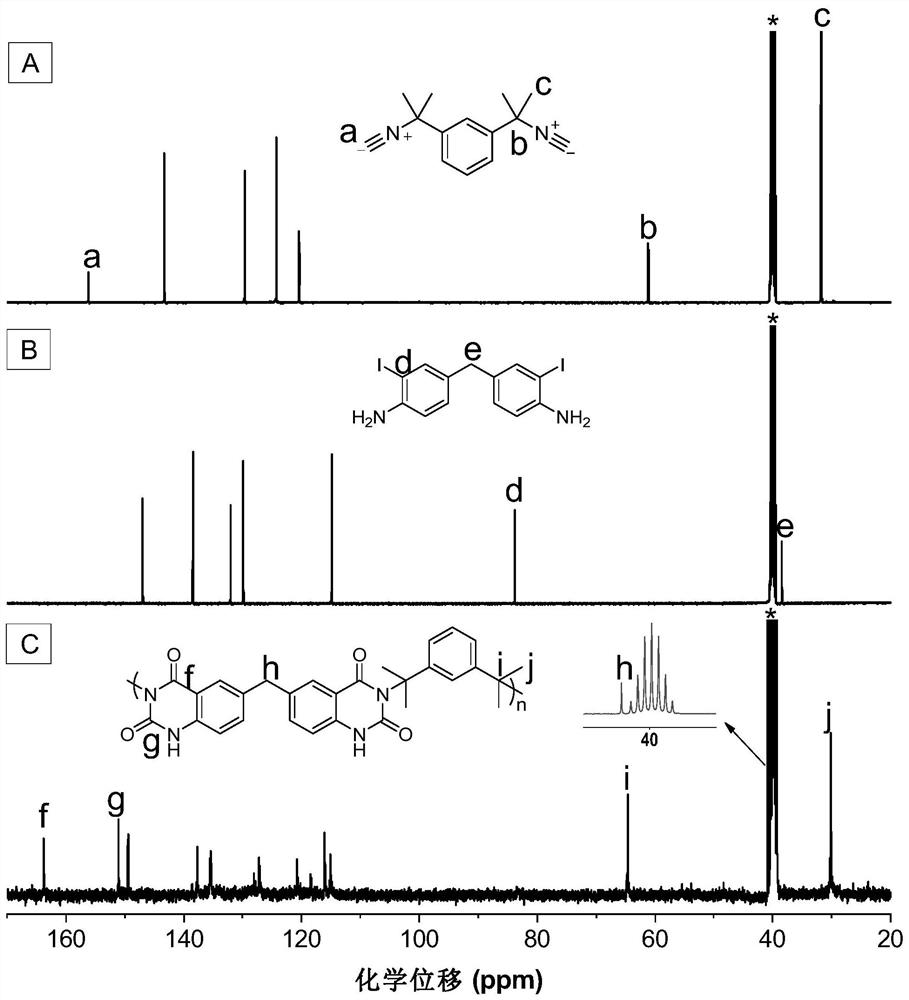
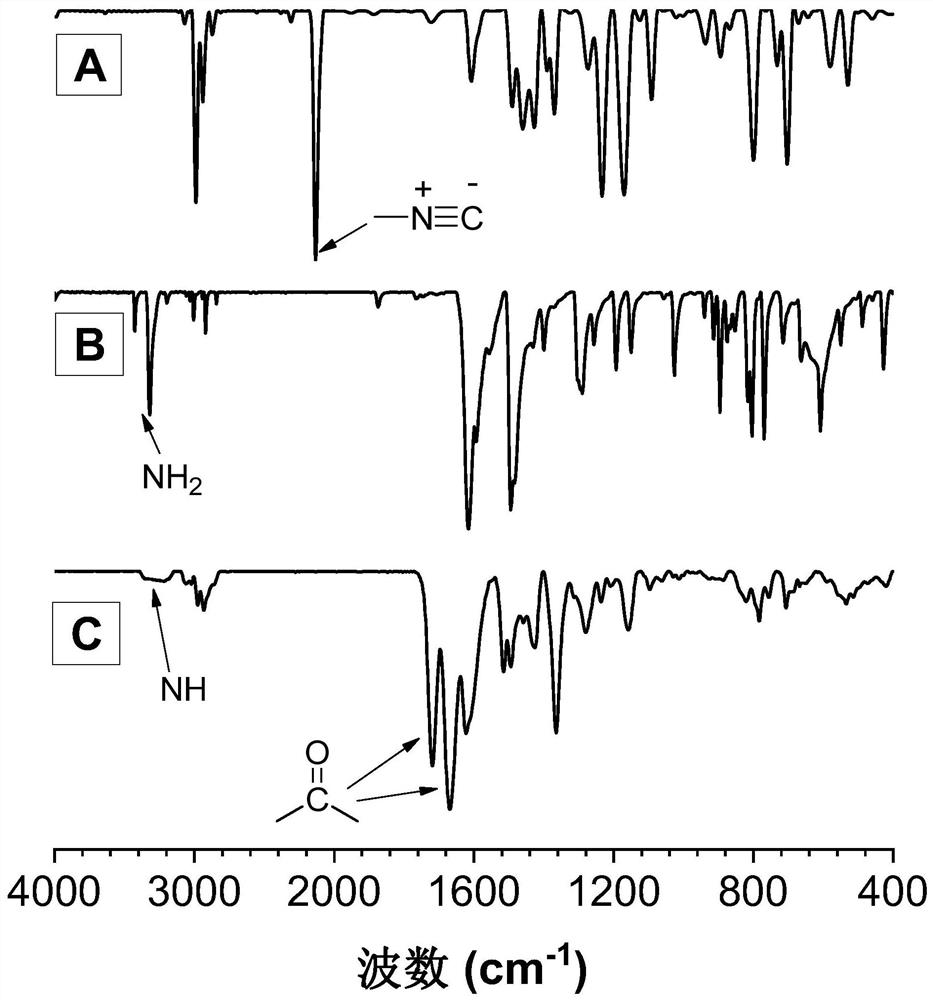
[0045] A kind of polyquinazoline compound, its structural formula is as shown in P1:
[0046]
[0047] The polyquinazoline compounds pass through the CO 2 , the polyreaction of difunctional isonitrile and difunctional o-iodoaniline is prepared, and the reaction equation is as formula (1):
[0048]
[0049]Wherein, the synthesis method of monomer M1 can refer to the synthesis method of literature (RSC Adv., 2015,5(56):44722-44727); the synthesis method of monomer N1 can refer to literature (J.Am.Chem.Soc. , 2018,140(19):6156-6163) synthetic method.
[0050] The preparation steps of described polyquinazoline compounds are as follows:
[0051] Add M1 (45mg, 0.1mmol), PdCl 2 (20mol%) and PPh 3 (40mol%), evacuated for 20 minutes, plunged into a filled with CO 2 Inject 0.5mL N,N-dimethylacetamide (DMAc) into the balloon, and after dissolving, dissolve N1 (21.3mg, 0.1mmol) and DBU (400mol%) with 0.5mL and 1mL DMAc respectively, and inject them into the reaction system in t...
Embodiment 2
[0054] A kind of polyquinazoline compound, its structural formula is as shown in P2:
[0055]
[0056] The polyquinazoline compounds pass through the CO 2 , the polyreaction of difunctional isonitrile and difunctional o-iodoaniline is prepared, and the reaction equation is as formula (two):
[0057]
[0058] Wherein, the synthesis method of monomers M2 and N1 is the same as in Example 1.
[0059] The preparation steps of described polyquinazoline compounds are as follows:
[0060] Add M2 (464mg, 1mmol), PdCl 2 (30mol%) and PPh 3 (60mol%), evacuated for 20 minutes, plunged into a CO 2 Inject 0.5mL N,N-dimethylacetamide (DMAc) into the balloon, and after dissolution, dissolve N1 (212.5mg, 1mmol) and Cs with 0.5mL and 1mL DMAc respectively 2 CO 3 (500mol%), injected into the reaction system in turn, reacted at 80°C for 2h, then cooled to room temperature, added 3mL of DMAc to dissolve, passed the obtained polymer solution through a cotton filter device, and added drop...
Embodiment 3
[0063] A kind of polyquinazoline compound, its structural formula is as shown in P3:
[0064]
[0065] The polyquinazoline compounds pass through the CO 2 , the polyreaction of difunctional isonitrile and difunctional o-iodoaniline is prepared, and the reaction equation is as formula (three):
[0066]
[0067] Wherein, the synthesis method of monomers M3 and N1 is the same as in Example 1.
[0068] The preparation steps of described polyquinazoline compounds are as follows:
[0069] Add M3 (245.6mg, 0.4mmol), PdCl 2 (5mol%) and PPh 3 (10mol%), evacuated for 20 minutes, plunged into a CO 2 Inject 0.5mL N,N-dimethylacetamide (DMAc) into the balloon, and after dissolution, dissolve N1 (85.2mg, 0.4mmol) and CS with 0.5mL and 1mL DMAc respectively 2 CO 3 (600mol%), injected into the reaction system in turn, reacted at 50°C for 8h, then cooled to room temperature, added 3mL of DMAc to dissolve, passed the obtained polymer solution through a cotton filter device, and adde...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com