Polyquinoline diketone compound as well as preparation method and application thereof
A technology for polyquinoline diones and compounds, which is applied in the fields of polymer chemistry and materials science, can solve the problems of little research on the construction of heterocyclic polymers, and achieve the effects of high thermal stability and refractive index, low cost and simple operation.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
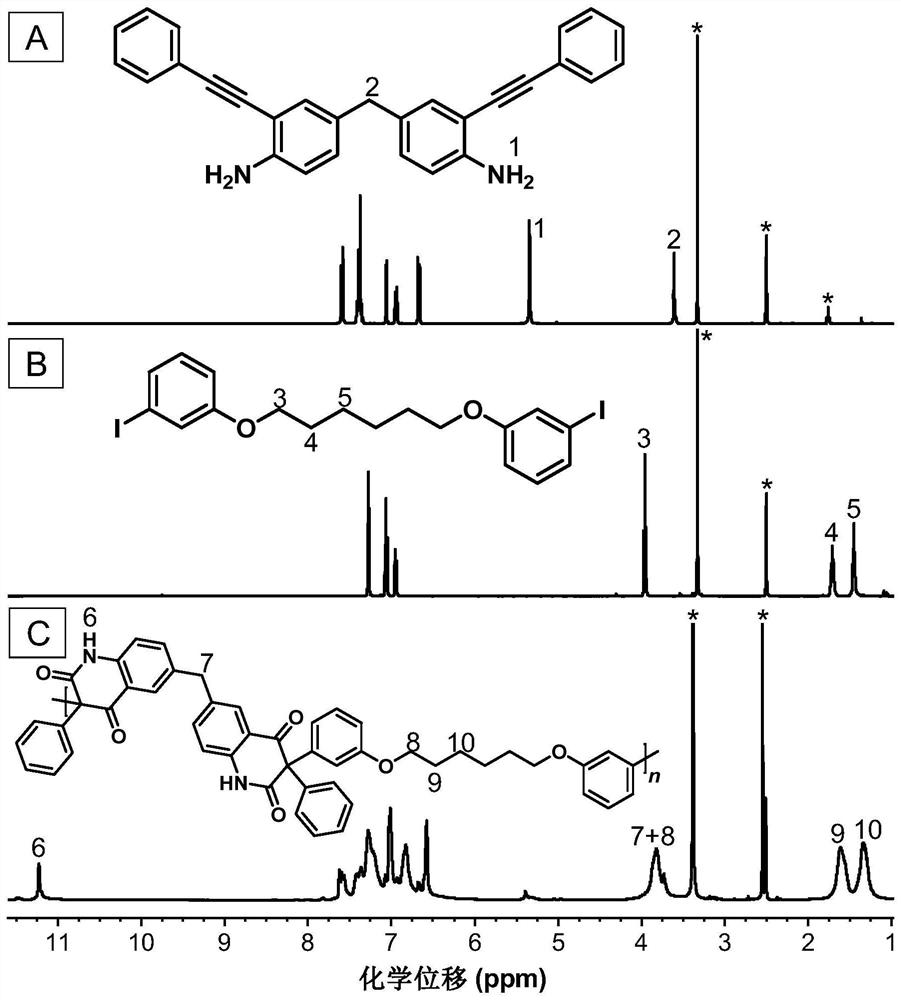
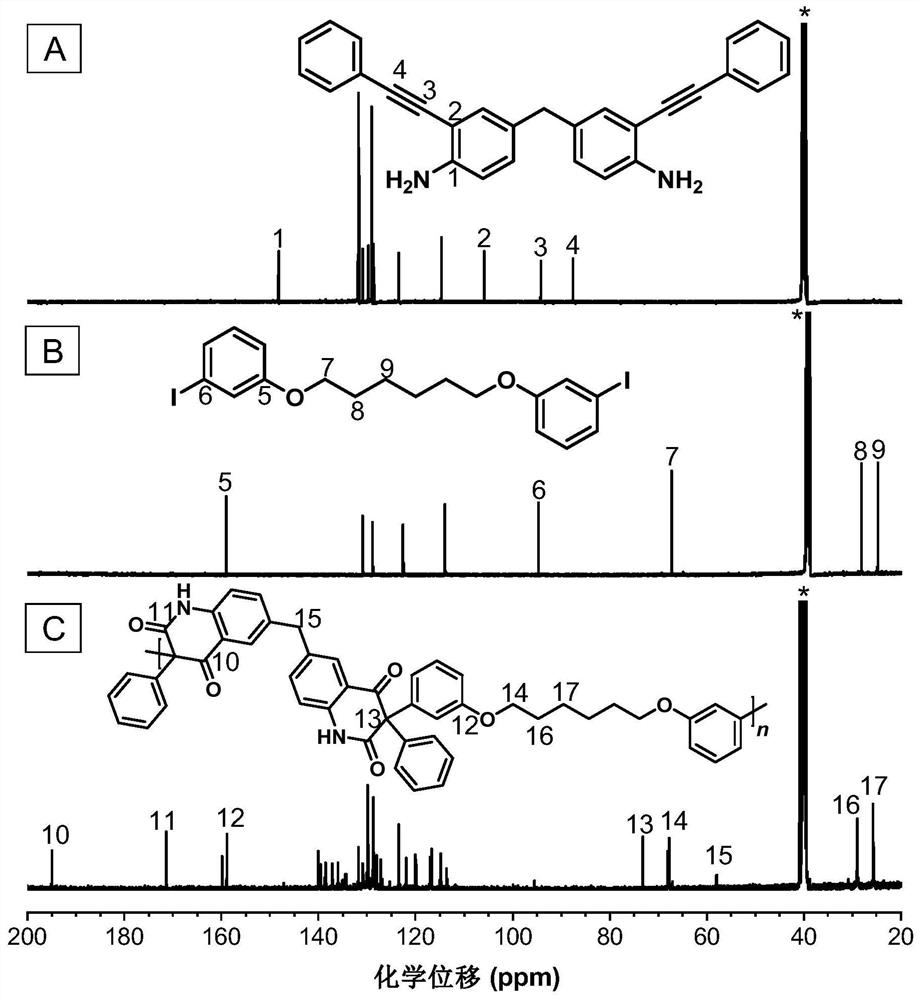
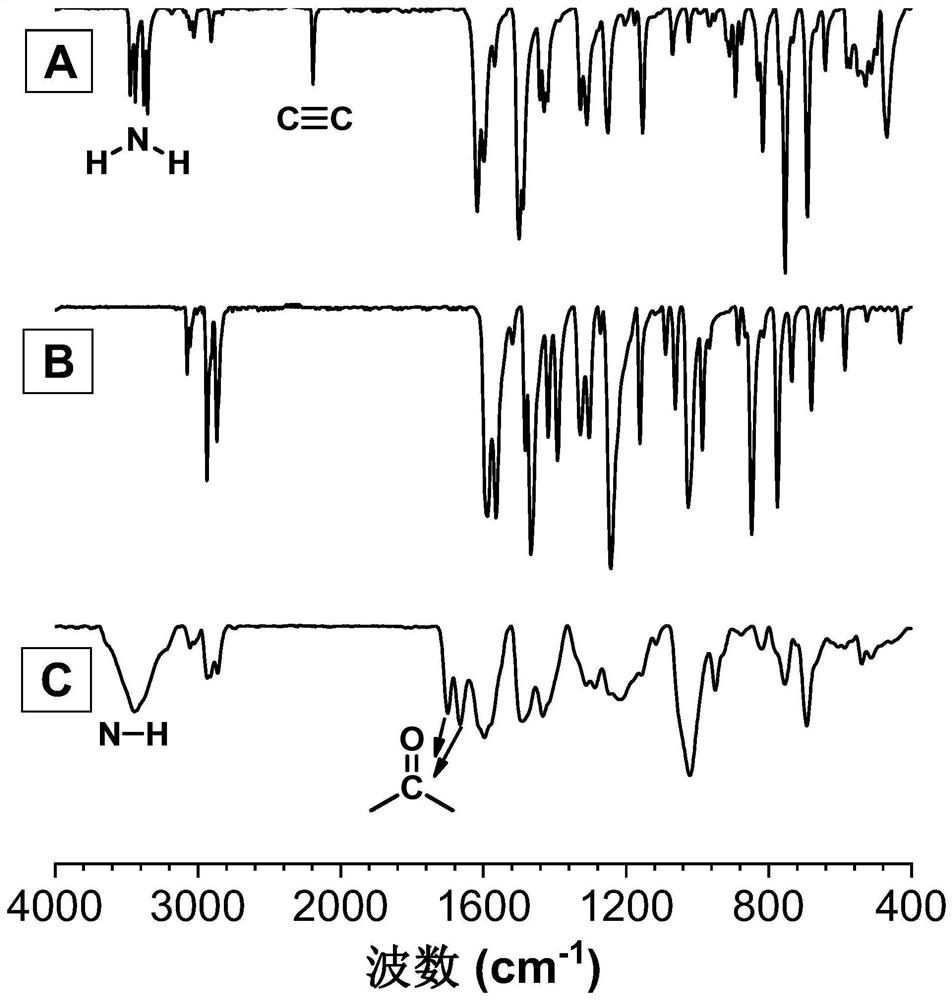
[0048] A kind of polyquinoline dione compound, its structural formula is as shown in P1:
[0049]
[0050] The polyquinoline diones are passed through CO 2, the polyreaction of bifunctional group o-alkyne aniline monomer and bifunctional aryl iodide monomer is prepared, and the reaction equation is as formula (1):
[0051]
[0052] Wherein, o-iodoaniline intermediates can be synthesized with reference to the preparation method of literature (Macromolecules, 2021,54(9):4112-4119), and then synthesize monomer M1 through Sonogashira reaction with phenylacetylene; monomer N1 can refer to literature ( Chem.Commun., 2019,55(47):6755-6758) synthesis.
[0053] The preparation steps of described polyquinolinedione compound are as follows:
[0054] Add M1 (0.1 mmol, 39.9 mg), N1 (0.1 mmol, 52.2 mg), bistriphenylphosphine palladium dichloride (Pd(PPh 3 ) 2 Cl 2 ) (0.02mmol, 14mg) and cesium carbonate (Cs 2 CO 3 ) (0.2mmol, 65.3mg), replace CO 2 three times in CO 2 Under at...
Embodiment 2
[0057] A polyquinolinedione compound, its structural formula is as shown in P2:
[0058]
[0059] The polyquinoline diones are passed through CO 2 , the polyreaction of bifunctional group o-alkyne aniline monomer and bifunctional aryl iodide monomer is prepared, and the reaction equation is as formula (two):
[0060]
[0061]
[0062] Wherein, the synthesis method of monomer M1 is the same as in Example 1; monomer N2 was purchased from Matrix Scientific Company.
[0063] The preparation steps of described polyquinolinedione compound are as follows:
[0064] Add M1 (0.1mmol, 39.9mg), N2 (0.08mmol, 33.8mg), Pd(PPh 3 ) 2 Cl 2 (0.02mmol, 14mg) and Cs 2 CO 3 (0.2mmol, 65.3mg), replace CO 2 three times in CO 2 Under atmosphere, 0.33 mL of ultra-dry DMSO was injected via syringe. The reaction system was reacted at 80° C. for 1 hour. After the reaction, cool to room temperature, add 2.6mL DMSO, stir and dilute. Then the polymer solution obtained is passed through a...
Embodiment 3
[0067] A kind of polyquinoline dione compound, its structural formula is as shown in P3:
[0068]
[0069] The polyquinoline diones are passed through CO 2 , the polyreaction of bifunctional group o-alkyne aniline monomer and bifunctional aryl iodide monomer is prepared, and the reaction equation is as formula (three):
[0070]
[0071] Wherein, the synthesis method of monomer M1 is the same as that in Example 1; monomer N3 was purchased from Shanghai Beide Pharmaceutical Technology Co., Ltd.
[0072] The preparation steps of described polyquinolinedione compound are as follows:
[0073] Add M1 (0.1mmol, 39.9mg), N3 (0.12mmol, 52.1mg), Pd(PPh 3 ) 2 Cl 2 (0.02mmol, 14mg) and Cs 2 CO 3 (0.2mmol, 65.3mg), replace CO 2 three times in CO 2 Under atmosphere, inject 2 mL of ultra-dry DMSO via syringe. The reaction system was reacted at 140° C. for 8 hours. After the reaction was completed, cool to room temperature, add 1 mL DMSO, and stir to dilute. Then the polymer ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com