Fusion gene PSMD9-RNF34 in dyskeratosis congenital, and application and detection kit of fusion gene PSMD9-RNF34
A PSMD9-RNF34, fusion gene technology, applied in the field of biomedicine, can solve the problems of poor treatment effect, poor prognosis, and loss of treatment timing for patients
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
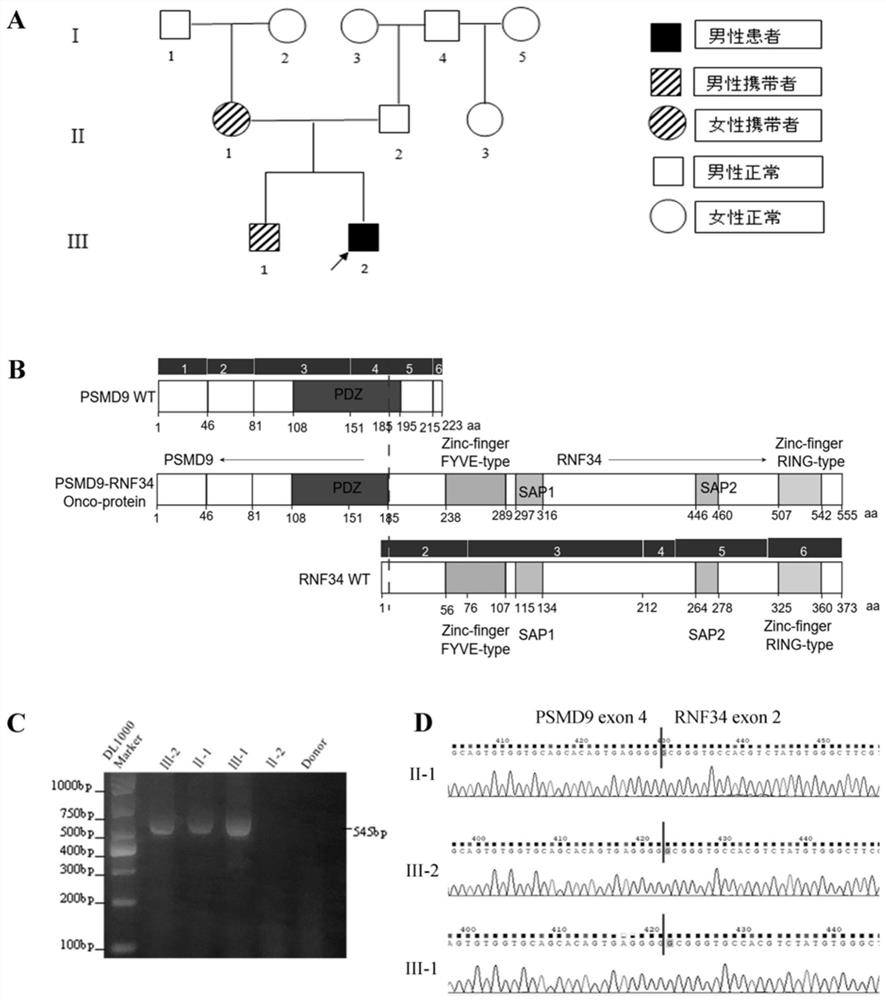
[0014] Example 1. Patients with dyskeratosis congenita and their family members carry PSMD9-RNF34 new fusion gene
[0015] 1. Using bioinformatics technology to screen a patient with dyskeratosis congenita, it was found that there was a fusion gene PSMD9-RNF34 fusion gene.
[0016] 2. Using PCR technology and performing sanger sequencing on the PCR product, we confirmed that the patient with dyskeratosis congenita carried the new PSMD9-RNF34 fusion gene. The specific CDS sequence is shown in SEQ ID NO.1, and the verification results are as follows figure 1 shown.
[0017] 3. Verify the fusion gene PSMD9-RNF34 in the family members of the patient with dyskeratosis congenita, and the verification results are as follows figure 1 shown.
Embodiment 2
[0018] Example 2. Preparation of PSMD9-RNF34 Fusion Gene Kit
[0019] 1. Specific primer design
[0020] According to the gene sequence (the PSMD9 gene sequence and the RNF34 gene sequence are all from the nucleic acid database of the National Biotechnology Information Center of the United States, the PSMD9 gene Entrez Gene ID 5717, the gene reference sequence NM_002813.7; the RNF34 gene Entrez Gene ID 80196, the gene reference sequence NM_194271.3 ) to design specific primers.
[0021] The primer sequences are as follows:
[0022] PSMD9-RNF34-F: GATGAACGAGCCGCTGGT (SEQ ID NO. 2);
[0023] PSMD9-RNF34-R: AGGTGGAAAACTCAGGGT (SEQ ID NO. 3).
[0024] 2. cDNA first-strand synthesis reagent: FastQuant RT Kit (TIANGEN, KR106), its main components include genomic DNA removal system, 10× Fast RT Buffer, RT Enzyme Mix, RT Primer Mix. Detection system PCR reaction solution: (TOYOBO, KOD-401), its main components include high-fidelity enzyme KOD-Plus-Neo, 10×PCR Buffer forKOD-Plus-Ne...
Embodiment 3
[0025] Example 3. The operation process of this kit detection
[0026] 1. Take the anticoagulant blood samples of the patients submitted for inspection, and extract the total RNA in the blood: add 1ml of red blood cell lysate to a clean 1.5ml centrifuge tube, and add 0.5ml of anticoagulant blood to mix well; stand at room temperature for 10 minutes; Centrifuge at 5,000rpm for 5min, discard the supernatant, and collect the cells at the bottom; add 0.5ml red blood cell lysate again, centrifuge at 5,000rpm for 5min, discard the supernatant, and collect the cells at the bottom; add 1ml TRIzol to the cells, pipette repeatedly until the precipitate is completely dissolved, Let stand at room temperature for 5 minutes; add 0.2ml chloroform, shake evenly; centrifuge at 14,000rpm 4°C for 10 minutes, absorb the supernatant, and transfer to another new centrifuge tube; add an equal volume of isopropanol, mix well up and down, and let stand at room temperature 10min; centrifuge at 14,000rp...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com