Preparation method of bimetallic oxide, bimetallic oxide and application
A bimetallic oxide and bimetallic technology, applied in the direction of metal/metal oxide/metal hydroxide catalyst, rare earth metal oxide/hydroxide, rare earth metal compound preparation/treatment, etc., can solve the problem of metal oxidation Problems such as powder particle size, electrical conductivity, catalytic activity, uneven composite metal oxide powder, large grain size, etc., to reduce the number of crystallization nuclei, improve the degree of porosity and specific surface area, and prevent the pre-calcination temperature from being too high Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
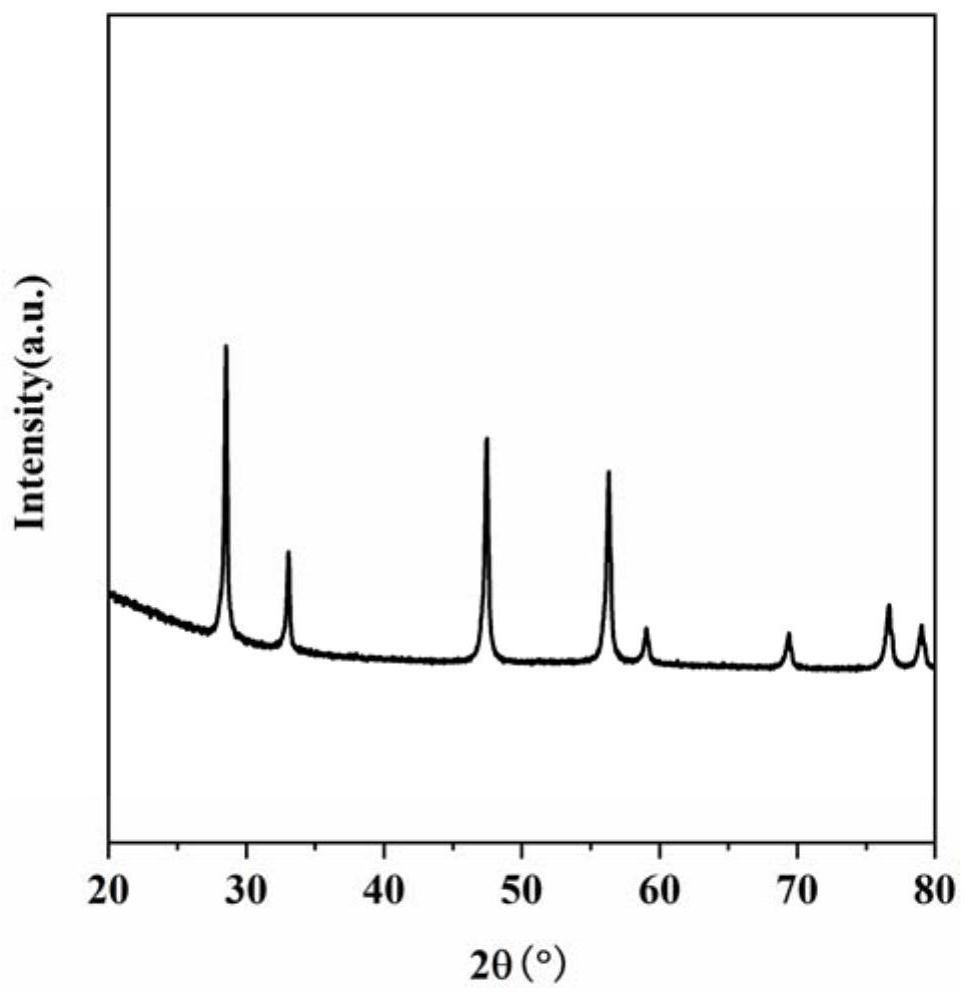
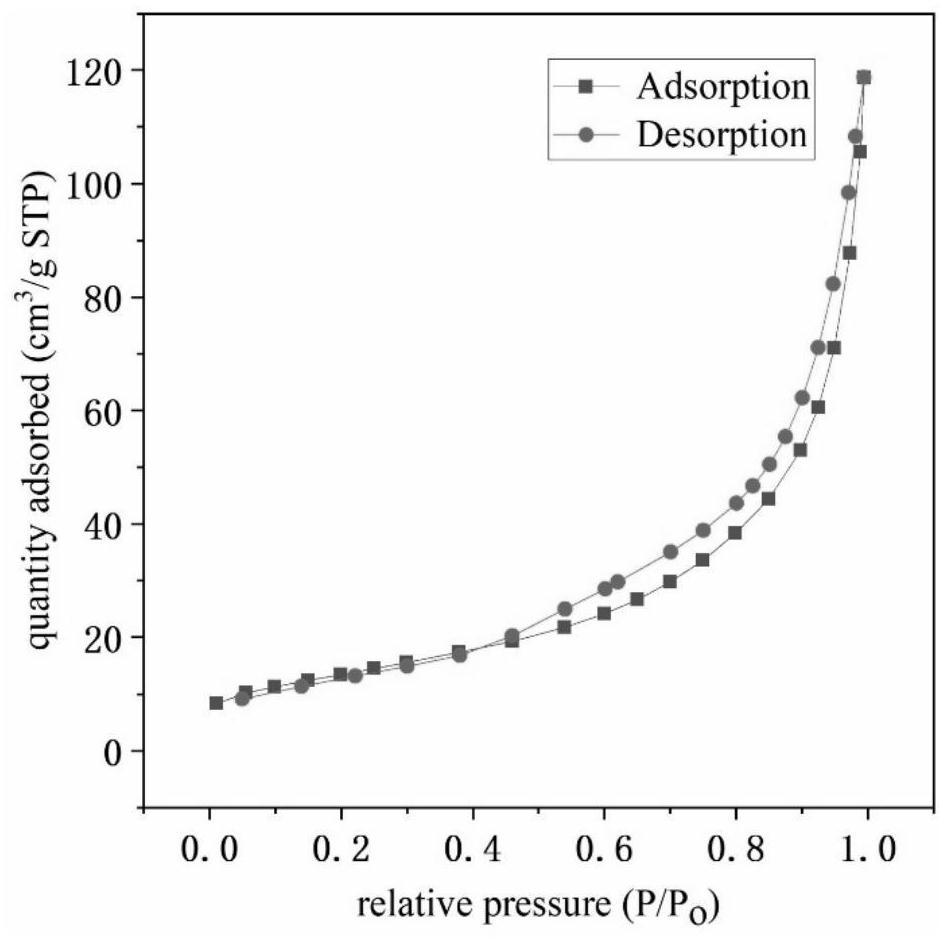
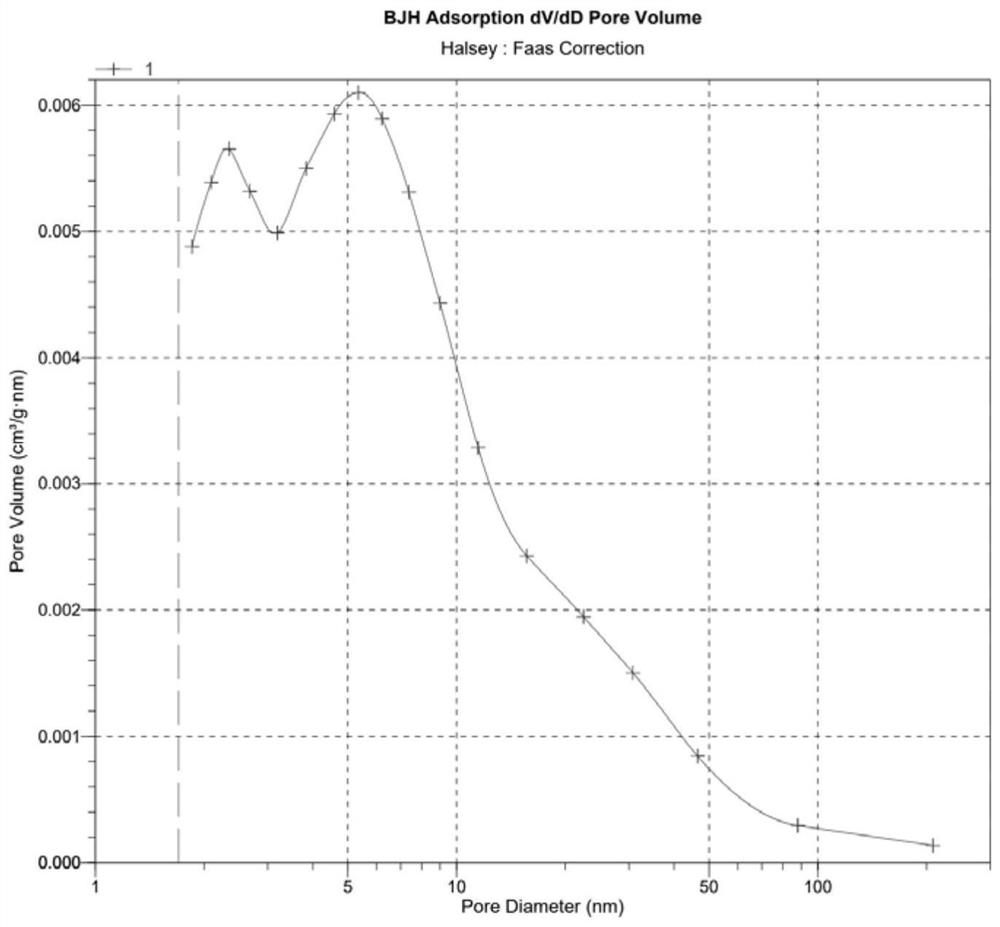
Image
Examples
preparation example Construction
[0025] A method for preparing a double metal oxide according to an embodiment of the present invention includes the following steps:
[0026] A, preparation samarium, cerium metal element mol ratio are (0.9-1.1): the bimetallic solution of 4;
[0027] b. Under stirring conditions, add ammonia water to the bimetallic solution to generate co-precipitates and separate them;
[0028] c. Drying, pre-calcining, calcining and cooling the co-precipitate in sequence, wherein the calcining temperature is 550-650°C.
[0029] According to the preparation method of a double metal oxide according to the embodiment of the present invention, 1. The precipitate obtained by the preparation method of the embodiment of the present invention is the precursor of the double metal oxide. Since ammonia water is used as the precipitant, part of the ammonia is accompanied by metal hydrogen The oxide precipitates and dissolves in the free water in the precursor. In the preparation method of the embodime...
Embodiment 1
[0047] a. According to the molar ratio of samarium and cerium metal elements being 1:4, weigh a considerable amount of Sm(NO 3 ) 3 ·6H 2 O, Ce(NO 3 ) 3 ·6H 2 O was dissolved in water respectively to obtain a samarium nitrate solution and a cerium nitrate solution, and the samarium nitrate solution was added dropwise to the cerium nitrate solution, and the cerium nitrate solution was continuously stirred at room temperature with a magnetic stirrer during the dropping process until the formation of Transparent and clear mixed solution, after continuous stirring for 2 hours, add dispersant dropwise at a rate of 10-12 drops / min directly above the stirring center, the molar ratio of dispersant to the total amount of bimetallic ions is 1:38, and bimetallic solution;
[0048] b. After adding the dispersant dropwise and stirring for 10 minutes, add 0.1mol / L ammonia water dropwise to the bimetallic solution under stirring conditions, and a milky white precipitate appears. Stop add...
Embodiment 2
[0051] a. According to the molar ratio of samarium and cerium metal elements being 1:4, weigh a considerable amount of Sm(NO 3 ) 3 ·6H 2 O, Ce(NO 3 ) 3 ·6H 2 O was dissolved in water respectively to obtain a samarium nitrate solution and a cerium nitrate solution, and the samarium nitrate solution was added dropwise to the cerium nitrate solution, and the cerium nitrate solution was continuously stirred at room temperature with a magnetic stirrer during the dropping process until the formation of Transparent and clear mixed solution, after continuous stirring for 2 hours, add dispersant dropwise at a rate of 10-12 drops / min directly above the stirring center, the molar ratio of dispersant to the total amount of bimetallic ions is 1:45, to obtain bimetallic solution;
[0052] b. After adding the dispersant dropwise and stirring for 10 minutes, add 0.1mol / L ammonia water dropwise to the bimetallic solution under stirring conditions, and a milky white precipitate appears. St...
PUM
| Property | Measurement | Unit |
|---|---|---|
| specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com