Raw material preparation method and detection method of vascular endothelial growth factor detection kit
A technology for detecting kits and growth factors, which is applied in the field of vascular endothelial growth factor detection, can solve problems such as the inability to quickly provide a disease control plan, the unfavorable mass production of kits, and the reduction of data repeatability and authenticity. , achieve the detection range and shorten the storage time, avoid non-specific binding and high binding efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
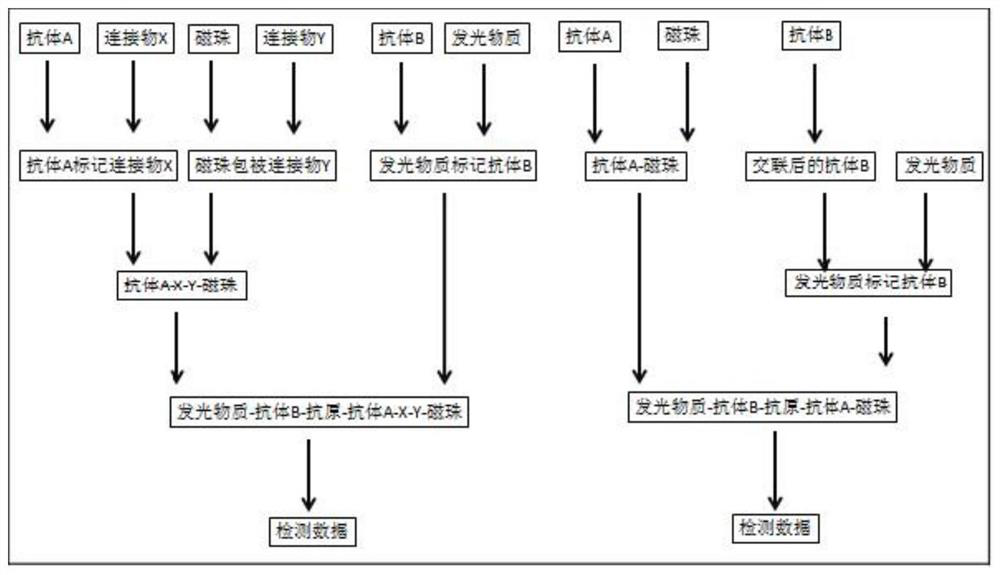
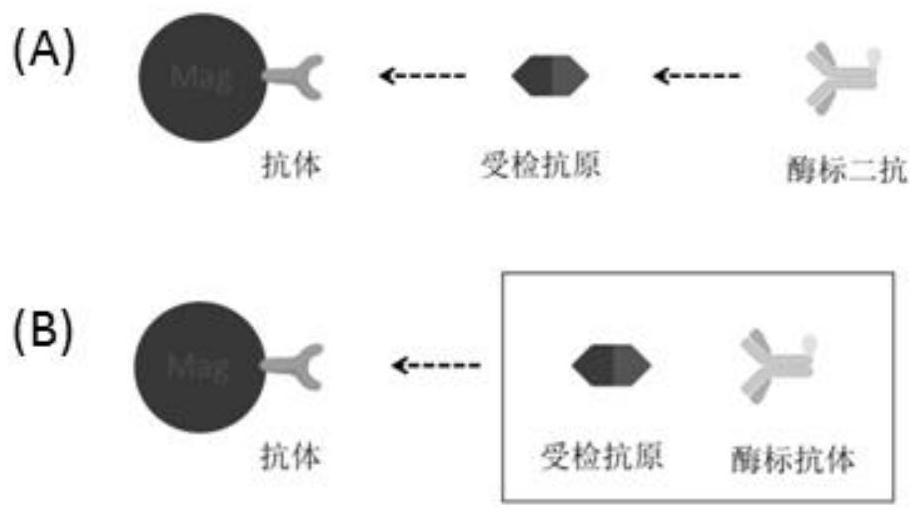
[0022] see Figure 4 , the raw material preparation and detection method of a vascular endothelial growth factor detection kit provided in this specific embodiment include:
[0023] (1) Raw material preparation, which includes antibody-coated magnetic bead working solution, VEGF-labeled antibody, VEGF calibrator, VEGF quality control product, and sample diluent;
[0024] The antibody-coated magnetic bead working solution is a suspension of magnetic microspheres coated with the Fab fragment of the humanized VEGF antibody, and the buffer system is TBS-T (20-200mM Tris, 0-1M NaCl, 0.0%-1%BSA, 0.1% -0.5% Tween-20, 0.1%-1% Proclin-300, pH 6.5-9.0), the preparation method is to take 200 μl of magnetic bead mother solution, add 0.1M borate buffer and 200 μg of recombinant VEGF antibody Fab fragment after resuspending Shake and mix well, and the magnetic beads are tosyl-activated magnetic beads, which can simultaneously react with the sulfhydryl and amino groups of the ligand without...
Embodiment 2
[0032] The main product performance indicators of a vascular endothelial growth factor detection kit are:
[0033] (1) Accuracy test of the kit
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com