Terbutaline sulfate oral liquid and production method thereof
A technology of terbutaline sulfate and its production method, which can be applied in the fields of pharmaceutical formula, dispersion liquid delivery, sanitary equipment for toilets, etc. It can solve the problems of increasing impurity content, affecting product quality, and product color darkening, etc., so as to reduce impurities The production of impurities, the effect of reducing the content of impurities and increasing the content
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
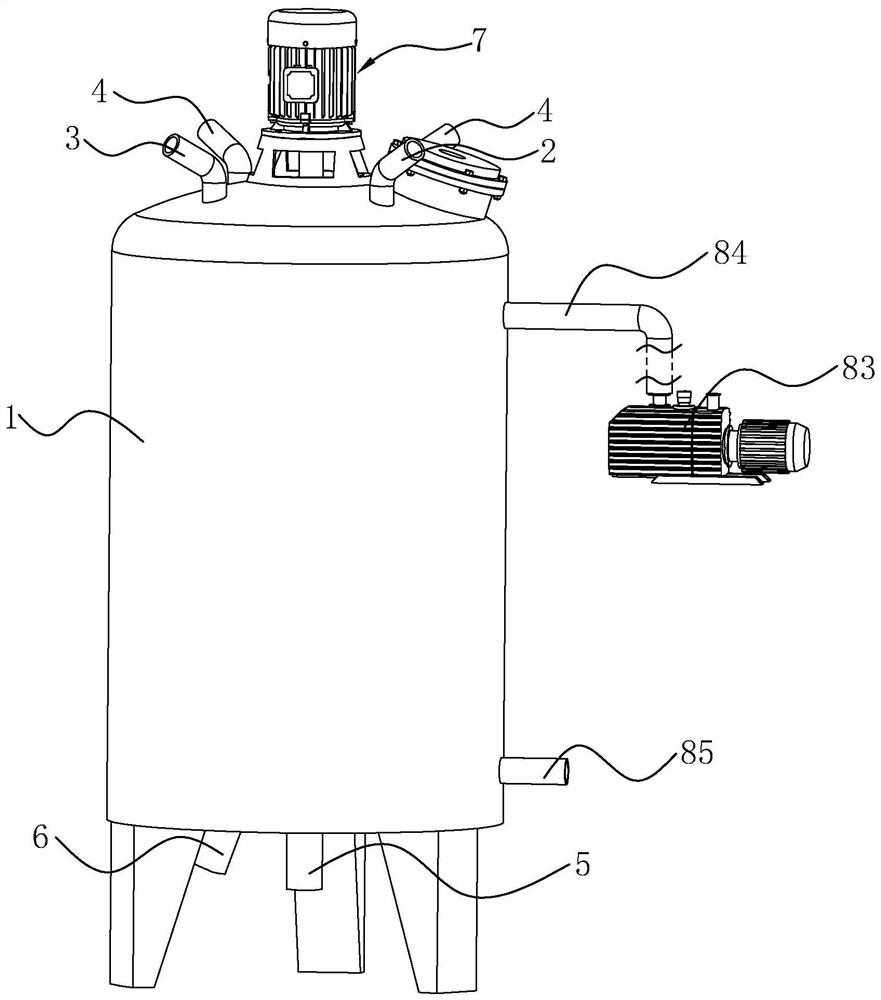
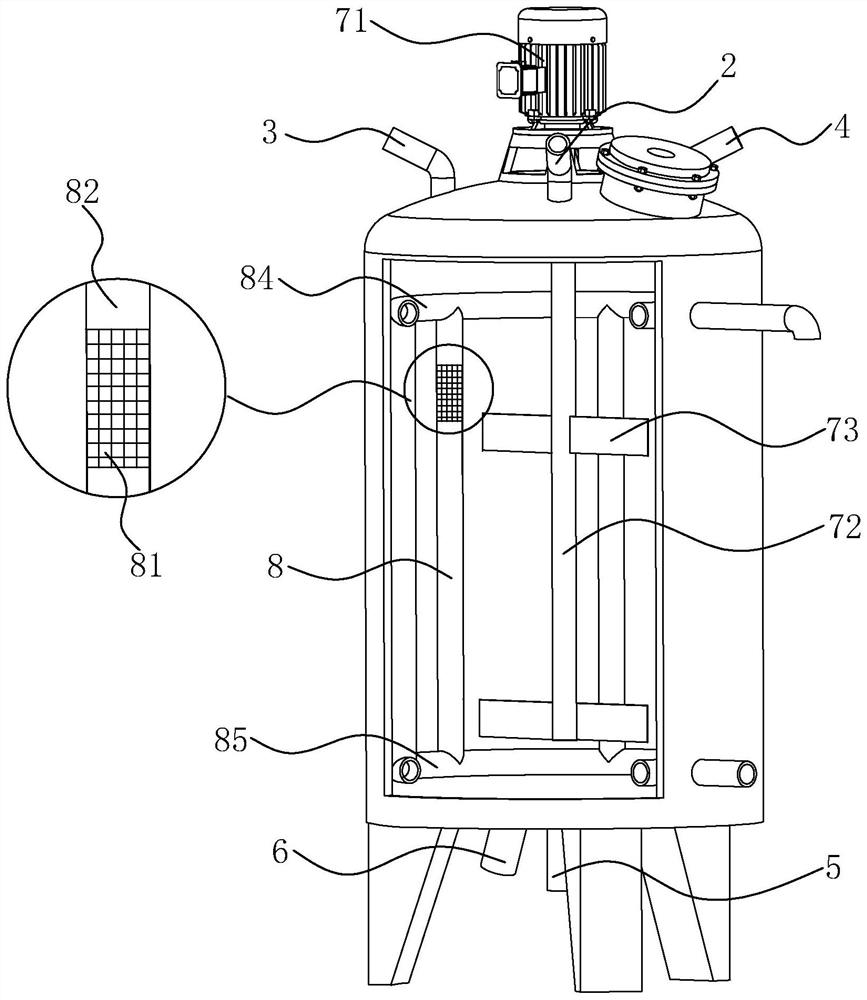
[0046] Embodiment 1, a kind of terbutaline sulfate oral liquid, the selection of each raw material component and its corresponding content are as shown in Table 1, and adopt following liquid preparation tank to prepare, and this liquid preparation tank comprises tank body 1, tank body The top of 1 is connected with feed pipe 4, liquid inlet pipe, vacuum suction pipe 2 and the first nitrogen filling pipe 3, the top of tank 1 is also provided with a manhole, and the bottom of tank 1 is connected with discharge pipe 5 and the second nitrogen filling pipe. Pipe 6, each pipe is provided with a control valve, and the tank body 1 is provided with a stirring assembly 7 and several deoxidation pipes 8. The stirring assembly 7 includes a stirring rod 72 , a stirring blade 73 arranged at the bottom of the stirring rod 72 , and a motor 71 for driving the stirring rod 72 .
[0047] Several deoxygenation tubes 8 are distributed in the tank body 1 in a circumferential shape around the stirri...
Embodiment 2~3
[0054] Embodiments 2-3, a terbutaline sulfate oral liquid, differ from Embodiment 1 in that the selection of each raw material component and its corresponding content are shown in Table 1.
[0055] Components and consumption (kg) of terbutaline sulfate oral liquid in table 1, embodiment 1~3
[0056]
Embodiment 4
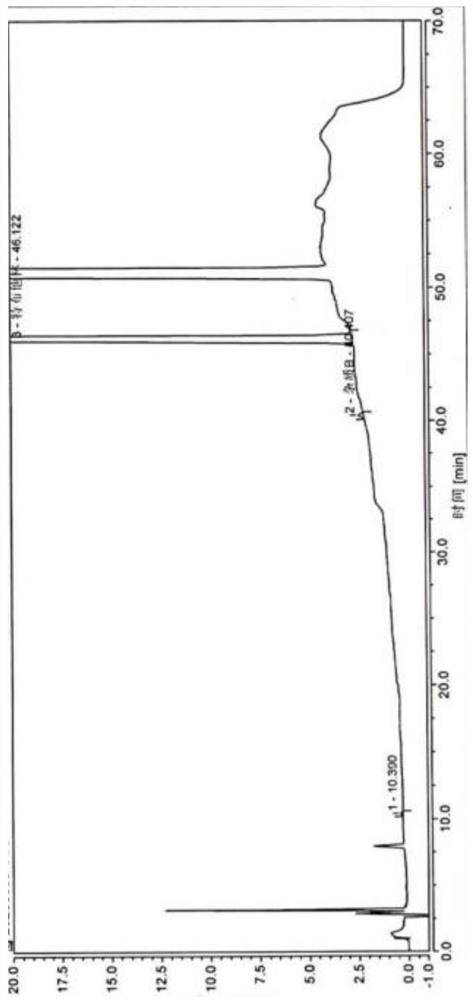
[0057] Example 4, a terbutaline sulfate oral liquid, differs from Example 1 in that in step S1, the vacuum pump 83 connected to the deoxygenation pipe 8 is not turned on during the stirring process, that is, the dissolved oxygen is not removed.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com