Composition of main ingredients for treating carbuncle, burn and acne, sustained and controlled release pharmaceutical preparation, preparation method and application thereof
A technology for burns and scald composition, applied in the field of traditional Chinese medicine preparations, can solve the problems of poor use effect and use experience, inconvenience in life and work, poor patient compliance, etc., and achieves improved medication compliance, good bioavailability, and drug release. stable effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
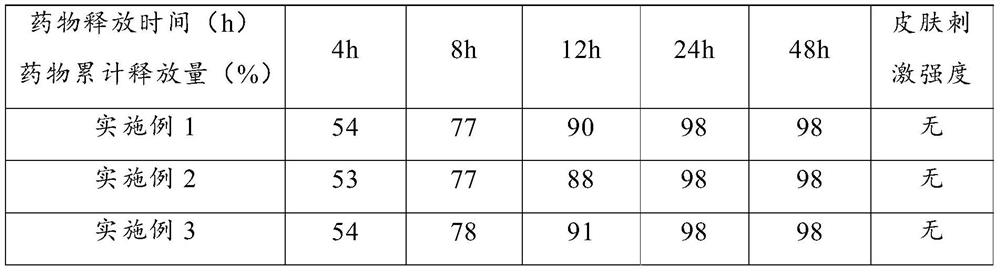
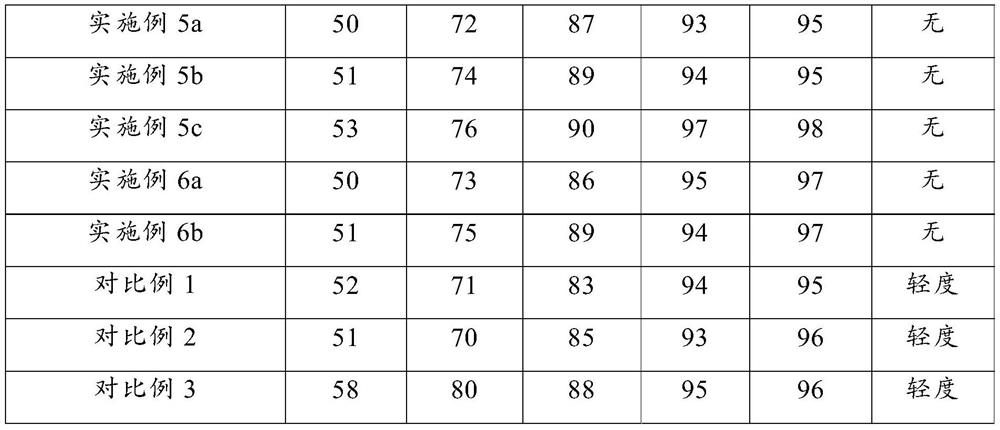
Examples
Embodiment 1
[0067] (I) Ingredients
[0068] I1-main ingredient composition:
[0069] Kochia extract, 5 parts by weight;
[0070] Centella asiatica extract, 2.5 parts by weight;
[0071] Borneol, 0.2 parts by weight.
[0072] I2-Liposome:
[0073] Phospholipids: soybean lecithin, 70 parts by weight;
[0074] Sterol compounds: cholesterol, 19.3 parts by weight;
[0075] Assistant emulsifier: propylene glycol, 2.4 parts by weight; Butanol, 0.6 parts by weight;
[0076] The first solvent: diethyl ether, 1000 parts by weight.
[0077] I3-substrate:
[0078] Water-based gel compound: hydroxyethyl cellulose, 1 part by weight; carbomer, 2 parts by weight
[0079] Preservative: methylparaben, 0.1 parts by weight;
[0080] Metal ion chelating agent: disodium edetate, 0.05 parts by weight;
[0081] Osmotic pressure regulator: sodium chloride, 0.85 parts by weight;
[0082] Moisturizing agent: sodium hyaluronate, 1 part by weight; glycerin, 5 parts by weight;
[0083] The second solvent, ...
Embodiment 2
[0090] (I) Ingredients
[0091] I1-main ingredient composition:
[0092] Kochia scoparia extract, 7 parts by weight;
[0093] Centella asiatica extract, 4.5 parts by weight;
[0094] Borneol, 0.7 parts by weight.
[0095] I2-Liposome:
[0096] Phospholipids: soybean lecithin, 68.8 parts by weight;
[0097] Sterol compounds: cholesterol, 15 parts by weight;
[0098] Assistant emulsifier: propylene glycol, 3.2 parts by weight; 0.8 parts by weight of n-butanol;
[0099] The first solvent: diethyl ether, 1000 parts by weight.
[0100] I3-substrate:
[0101] Aqueous gel compound: hydroxyethyl cellulose, 0.9 parts by weight; Carbomer, 2.1 parts by weight
[0102] Preservative: methylparaben, 0.1 parts by weight;
[0103] Metal ion chelating agent: disodium edetate, 0.05 parts by weight;
[0104] Osmotic pressure regulator: sodium chloride, 0.85 parts by weight;
[0105] Moisturizing agent: sodium hyaluronate, 0.2 parts by weight; glycerin, 0.8 parts by weight;
[0106] T...
Embodiment 3
[0113] (I) Ingredients
[0114] I1-main ingredient composition:
[0115] Kochia scoparia extract, 6 parts by weight;
[0116] Centella asiatica extract, 3.5 parts by weight;
[0117] Borneol, 0.5 parts by weight.
[0118] I2-Liposome:
[0119] Phospholipids: soybean lecithin, 75 parts by weight;
[0120] Sterol compounds: cholesterol, 25 parts by weight;
[0121] Assistant emulsifier: propylene glycol, 4.3 parts by weight; 0.7 parts by weight of n-butanol;
[0122] The first solvent: diethyl ether, 1000 parts by weight.
[0123] I3-substrate:
[0124] Water-based gel compound: hydroxyethyl cellulose, 1.2 parts by weight; Carbomer, 2.80 parts by weight
[0125] Preservative: methylparaben, 0.1 parts by weight;
[0126] Metal ion chelating agent: disodium edetate, 0.05 parts by weight;
[0127] Osmotic pressure regulator: sodium chloride, 0.85 parts by weight;
[0128] Moisturizing agent: sodium hyaluronate, 0.5 parts by weight; glycerin, 3.5 parts by weight;
[0129...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pore size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com