Synthesis method of 4-chloro-2-cyano benzene sulfonyl chloride
A technology of cyanobenzenesulfonyl chloride and its synthesis method, which is applied in chemical instruments and methods, preparation of sulfonic acid, preparation of organic compounds, etc., can solve the problems of increasing the workload of drug research process and prone to safety production accidents, and achieves Short reaction time and post-treatment time, avoiding diazotization reaction, and simple raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
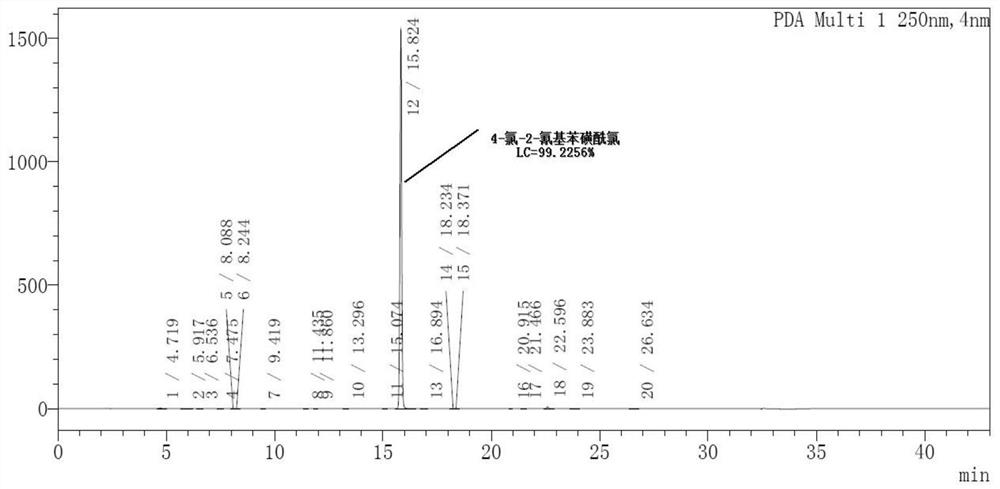
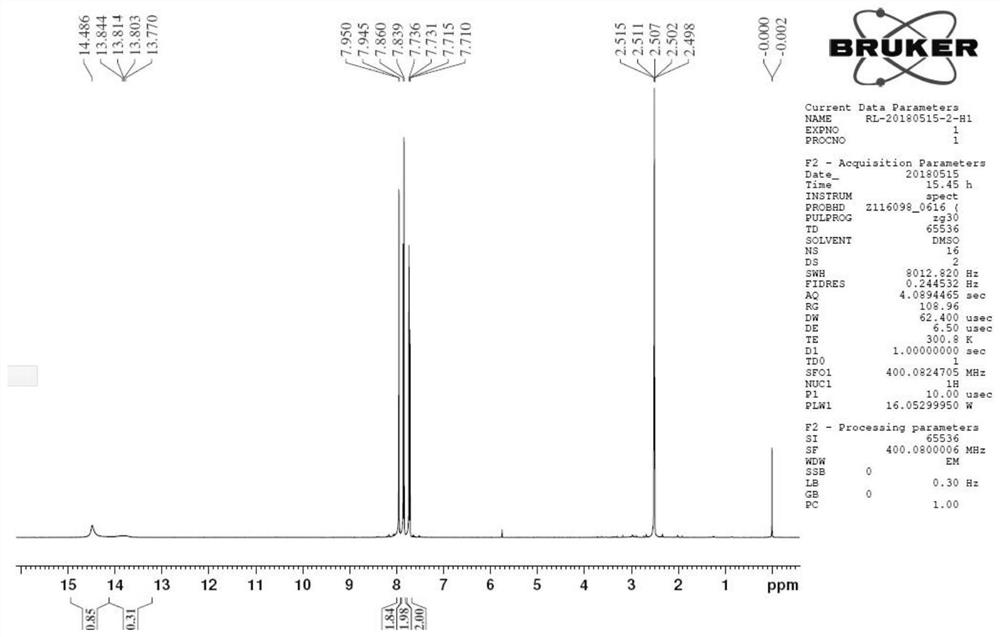
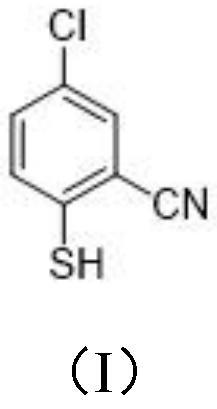
[0027] This example provides a synthetic method, detailed process and characterization analysis of 4-chloro-2-cyanobenzenesulfonyl chloride, including the synthetic method and detailed process of its synthetic precursor formula (I) compound and formula (II) compound, details as follows:
[0028] (1) Synthesis of the compound of formula (I)
[0029]
[0030] Under nitrogen protection, add 1500mL of DMF and 150.0g of 2-fluoro-5-chlorobenzonitrile to a 2000mL three-necked flask equipped with a mechanical stirrer and a thermometer. After stirring to dissolve and clarify, add Na 2 S·9H 2 O 277.91g, after adding the material, slowly heat up to 50°C-55°C and keep it warm for reaction. When 2-fluoro-5-chlorobenzonitrile GC<1%, stop the reaction.
[0031] After the reaction was completed, under the protection of nitrogen, the reaction liquid was naturally cooled to 20°C-25°C, poured into stirred 1200mL ice water, stirred for 5min, and the temperature was controlled at 10°C-20°C un...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com