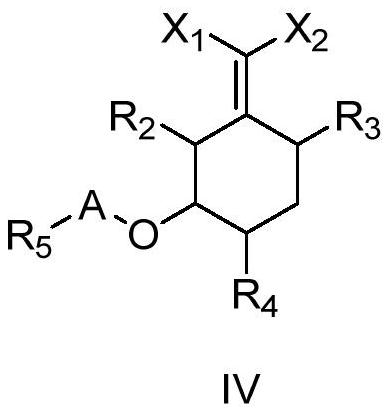
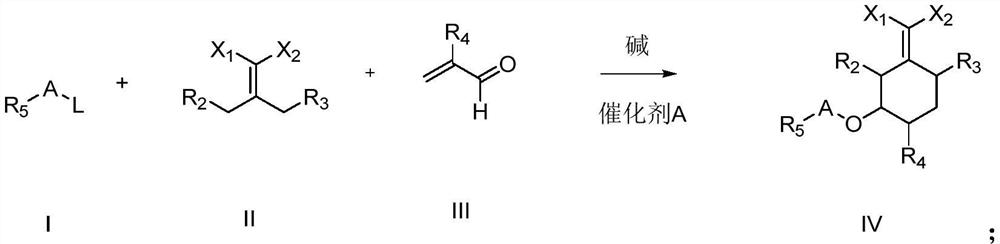
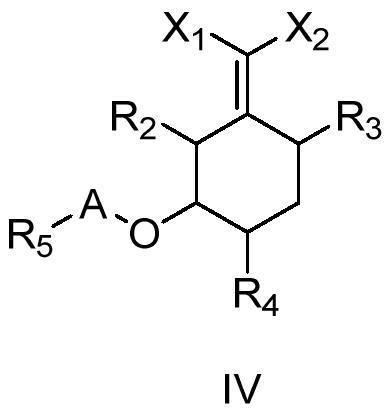
Cyclohexyl ester compound as well as preparation method and application thereof
A cyclohexyl ester and compound technology, applied in the field of organic synthesis, can solve the problems of complicated post-processing, high reaction temperature, participation of precious metals, etc., and achieve the effects of avoiding unfriendly environment, simple preparation and wide application prospect.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0073] Embodiment 1: the synthesis of 2-(4-heptyl) malononitrile
[0074] Add 30 g of 4-heptanone, 18.2 g of malononitrile, 3.05 g of ammonium acetate, 4.75 g of acetic acid and 200 mL of toluene into a three-necked flask equipped with a mechanical stirring device and a water separator, heat the system to 120 ° C, and separate the water for 2 hours. The total water content was 4.5 mL. The system was cooled down, washed with saturated brine, and concentrated to obtain crude 2-(4-heptylene)malononitrile, which was directly used in the next reaction without purification.
Embodiment 2
[0075] Embodiment 2: the synthesis of compound IV-1
[0076] Add 1.62 g (0.01 mol) of the crude product 2-(4-heptylethylene) malononitrile obtained in the above examples to 10 ml of anhydrous methanol, cool to 0° C., dropwise add 1.4 mL of 30% NaOMe solution and 2-methylpropene Aldehyde 0.7g, dropwise, stirred at room temperature for 1 hour. After concentrating under reduced pressure, add 20 mL of dichloromethane, 1.01 g of triethylamine, and 122 mg of DMAP to the oil, dropwise add 0.8 mL of acetyl chloride in an ice bath, keep warm for 2 hours in an ice bath, add water, extract and separate, and collect The organic phase was concentrated and subjected to silica gel column chromatography (petroleum ether: ethyl acetate = 10:1 (v / v)) to obtain 1.86 g of compound IV-1, yield: 67.8%. 1 H NMR (600MHz, CDCl 3 ):6.85(s,1H),3.07-3.03(m,1H),2.39-2.35(m,2H),2.23-2.19(m,1H),2.14(s,3H),2.12-2.10(m,1H ), 1.69(d,3H), 1.68-1.60(m,3H), 1.52-1.44(m,1H), 1.05(t,3H), 0.89(t,3H).
Embodiment 3
[0077] Embodiment 3: the synthesis of compound IV-1
[0078] Add 1.62g (0.01mol) of 2-(4-heptylene)malononitrile, 0.7g of 2-methacrolein, 1.01g of triethylamine, 122mg of DMAP and 20mL of 1,2-dichloroethane into the reaction flask 1.02 g of acetic anhydride was added with stirring at room temperature. After the addition was complete, the system was stirred overnight at room temperature. After adding water, extract and separate the layers, collect the organic phase, concentrate and perform silica gel column chromatography (petroleum ether: ethyl acetate = 10:1) to obtain 1.9 g of compound IV-1, yield: 69.2%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com