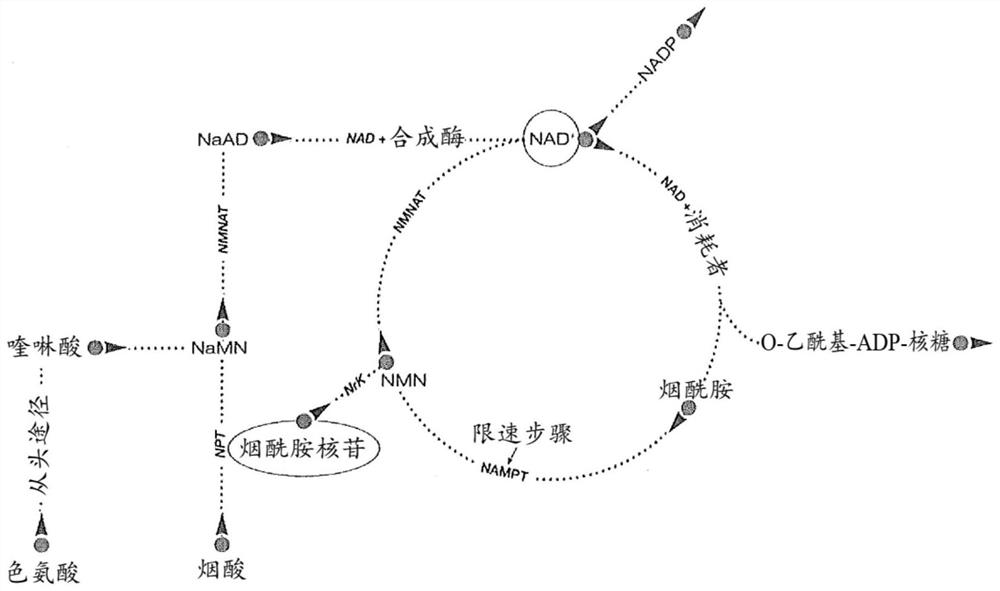
Use of nicotinamide riboside, nicotinic acid riboside, reduced nicotinyl riboside compounds, and nicotinyl riboside compound derivatives in formulations
A technology of nicotinyl riboside compound and nicotinamide riboside is applied in the field of product application of nicotinamide riboside, nicotinic acid riboside, reduced nicotinyl riboside compound and nicotinyl riboside compound derivative, and can solve the problem of Issues such as unreported quantitative effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
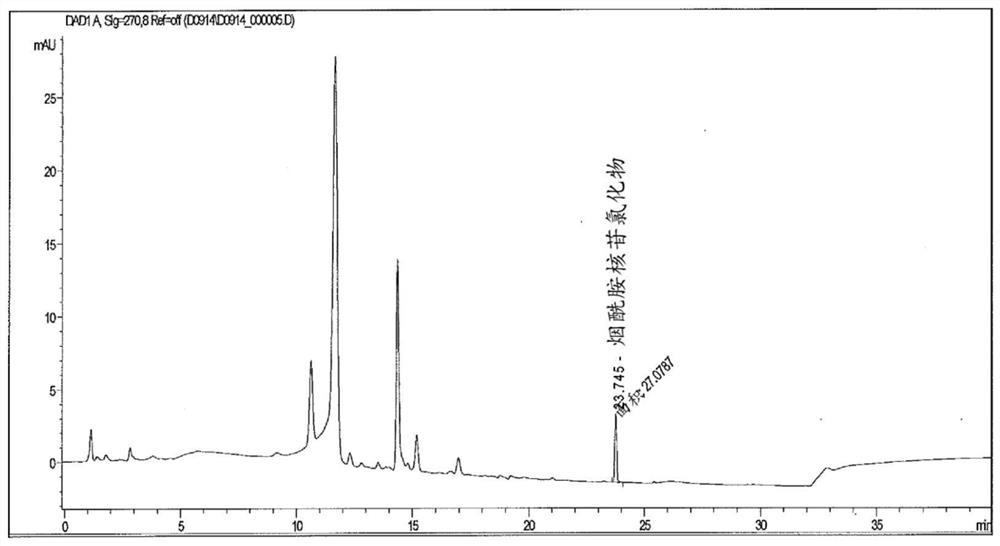
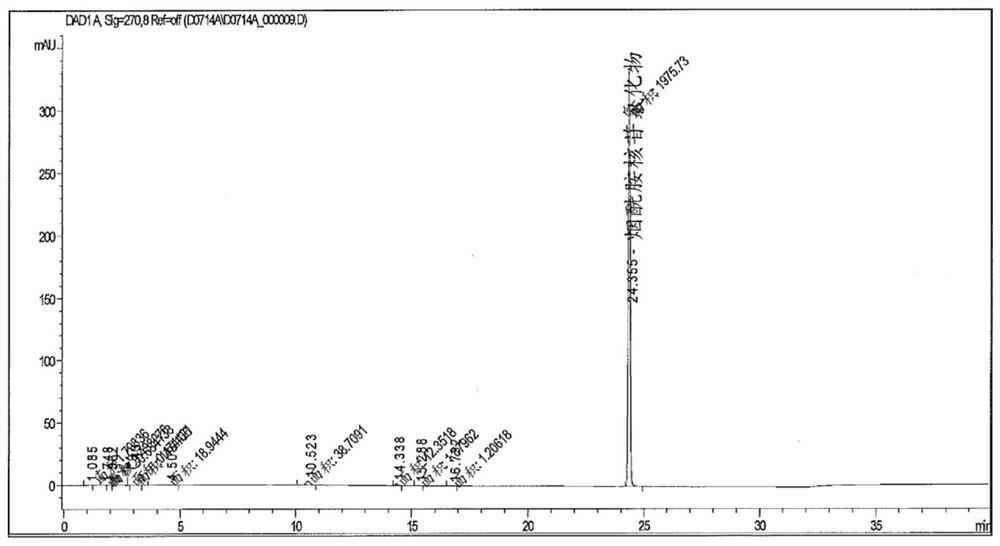
[0236] Nicotinamide riboside (NR, I) also occurs naturally in milk. Figure 2 shows the presence of nicotinamide riboside (NR, I) in store-bought (bovine) milk. Figure 2B and 2C is a control chromatogram showing detection of nicotinamide riboside (NR, I) after addition of nicotinamide riboside (NR, I) in known amounts to milk samples. These control chromatograms demonstrate that nicotinamide riboside (NR, I) can be added to milk and subsequently quantitatively recovered without significant degradation, or demonstrate that nicotinamide riboside (NR, I) is not compatible with commercial milk. The calculated recovery of 1% nicotinamide riboside (NR, I) was close to 100%. The experimental method used to obtain these results was as follows: Milk was diluted 1:1 with acetonitrile. Centrifugation was then performed to remove any precipitate and the supernatant analyzed using HILIC / HPLC / UV using standard methods.
[0237] Nicotinamide riboside (NR, I) also occurs naturally in huma...
example 2
[0241] A. Solutions of hydrolyzed whey protein isolate (>90% protein, 2%-7% degree of hydrolysis) were prepared at different concentrations and filtered through a 0.2 μm sterile filter. Aliquots of sterile nicotinamide riboside chloride ("NR-Cl", the salt of NR, I) in aqueous solution were spiked into the sterile whey solution, aiming for a final NR-Cl concentration of 1 mmol / liters (0.3 mg / mL). The solution containing the mixture of hydrolyzed whey and NR-Cl was filtered through a 10 kDa molecular weight cut-off centrifugal filter to remove whey protein (14-80 kDa) and any protein-bound NR-Cl. Any NR-Cl not bound to whey protein was recovered in the filtrate and quantified by HPLC. It was demonstrated that as the amount of whey in solution increased, the percentage of NR-Cl bound to whey protein increased (see Table 1).
[0242] Table 1
[0243] NR-Cl bound to hydrolyzed whey protein isolate as a function of whey to NR-Cl ratio
[0244]
[0245]
[0246] Solutions ...
example 3
[0304] Nicotinamide riboside (NR, I) is long-term unstable in water, and the bond linking the ribose sugar and the nicotinamide ring is very susceptible to hydrolysis in an aqueous liquid environment. Therefore, it is believed that by exploiting the tight chemical interaction between nicotinamide riboside (NR, I) and proteins or hydrocolloids in the powder preparation step, the ability of nicotinamide riboside (NR, I) in an aqueous environment can be demonstrated. Improved stability. It is further believed that nicotinamide riboside (NR, I) can be incorporated or encapsulated, allowing the expansion and manufacture of suitable nicotinamide riboside (NR, I) particle complexes, which show significant resistance to hydrolysis.
[0305] A. Nicotinamide riboside (NR, I) and protein binding by spray drying
[0306] Two protein sources will be chosen, dry hydrolyzed whey protein isolate and dry unhydrolyzed whey protein isolate. The starting solution will be prepared to a solids...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com