Acetazolamide sodium freeze-dried powder injection as well as preparation method and application thereof
A technology of acetazolamide sodium and freeze-dried powder injection, which is applied in the field of biomedicine, can solve problems such as the effectiveness and safety of clinical medication, certain risks in clinical application, and residual organic solvents in the final product, so as to achieve stable product quality, The effect of convenient preparation and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
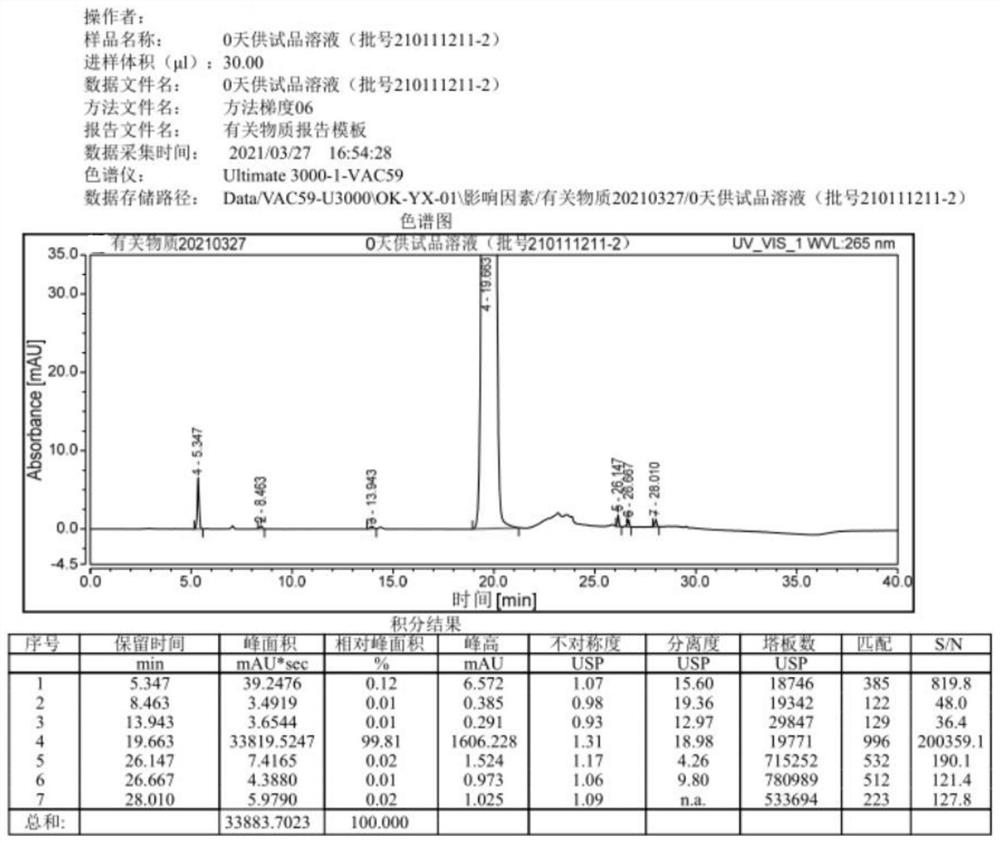
[0031] The prescription of this embodiment acetazolamide sodium freeze-dried powder injection is shown in Table 1.
[0032] Table 1
[0033] Acetazolamide 10.066kg sodium hydroxide 3.5kg Water for Injection Add to 106.6kg
[0034] A preparation method of acetazolamide sodium freeze-dried powder injection, comprising the following steps:
[0035] (1) Dosing: Inject 25kg of water for injection into a 200L dosing tank, cool down to 20°C, add 51kg of 5% sodium hydroxide solution, stir evenly, keep the temperature at 8°C, and then add 10.066kg of acetazolamide , stir and dissolve completely, measure the pH value, continue to add 5% sodium hydroxide solution to make the amount of sodium hydroxide reach the amount designed in the prescription, and adjust the pH value to 9.6±0.2, if the pH value is too high, you can add an appropriate amount Adjust with 0.1mol / L HCl, and continue to add water for injection to the prescribed amount to obtain a medicinal ...
Embodiment 2
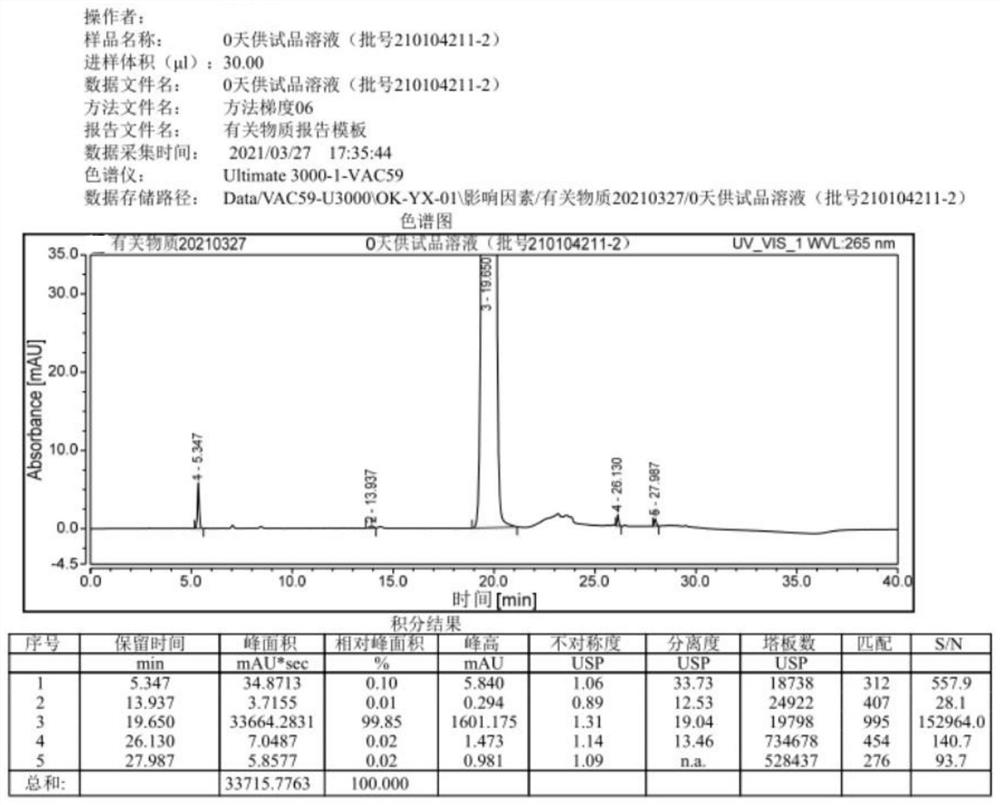
[0041] The prescription of this embodiment acetazolamide sodium freeze-dried powder injection is shown in Table 2.
[0042] Table 2
[0043] Acetazolamide 10.072kg sodium hydroxide 3.5kg Water for Injection Add to 106.76kg
[0044] A preparation method of acetazolamide sodium freeze-dried powder injection, comprising the following steps:
[0045](1) Dosing: Inject 25kg of water for injection into a 200L dosing tank, cool down to 10°C, add 51kg of 5% sodium hydroxide solution, stir evenly, keep the temperature at 5°C, and then add 10.072kg of acetazolamide , stir and dissolve completely, measure the pH value, continue to add 5% sodium hydroxide solution to make the amount of sodium hydroxide reach the amount designed in the prescription, and adjust the pH value to 9.6±0.2, if the pH value is too high, you can add an appropriate amount Adjust with 0.1mol / L HCl, and continue to add water for injection to the prescribed amount to obtain a medicinal ...
Embodiment 3
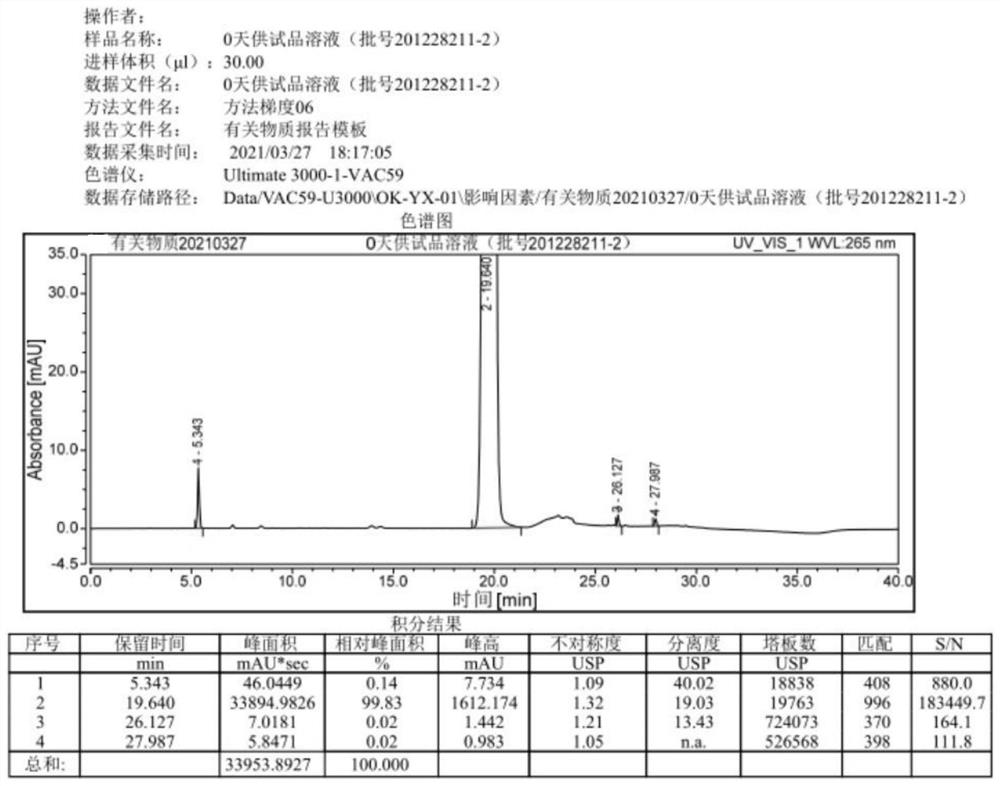
[0051] The prescription of this embodiment acetazolamide sodium freeze-dried powder injection is shown in Table 3.
[0052] table 3
[0053] Acetazolamide 10.069kg sodium hydroxide 3.5kg Water for Injection Add to 106.8kg
[0054] A preparation method of acetazolamide sodium freeze-dried powder injection, comprising the following steps:
[0055] (1) Dosing: Inject 25kg of water for injection into a 200L dosing tank, cool down to 30°C, add 51kg of 5% sodium hydroxide solution, stir evenly, keep the temperature at 10°C, and then add 10.069kg of acetazolamide , stir and dissolve completely, measure the pH value, continue to add 5% sodium hydroxide solution to make the amount of sodium hydroxide reach the amount designed in the prescription, and adjust the pH value to 9.6±0.2, if the pH value is too high, you can add an appropriate amount Adjust with 0.1mol / L HCl, and continue to add water for injection to the prescribed amount to obtain a medicinal...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com