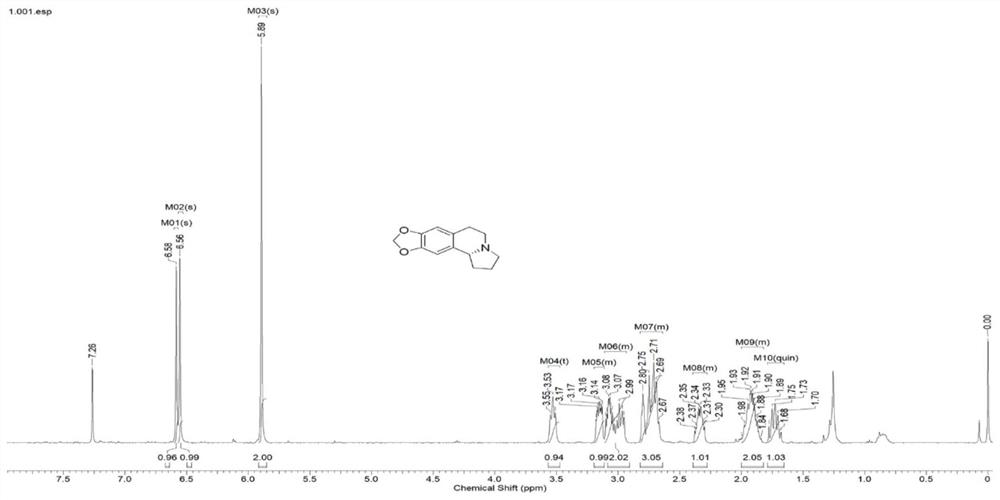
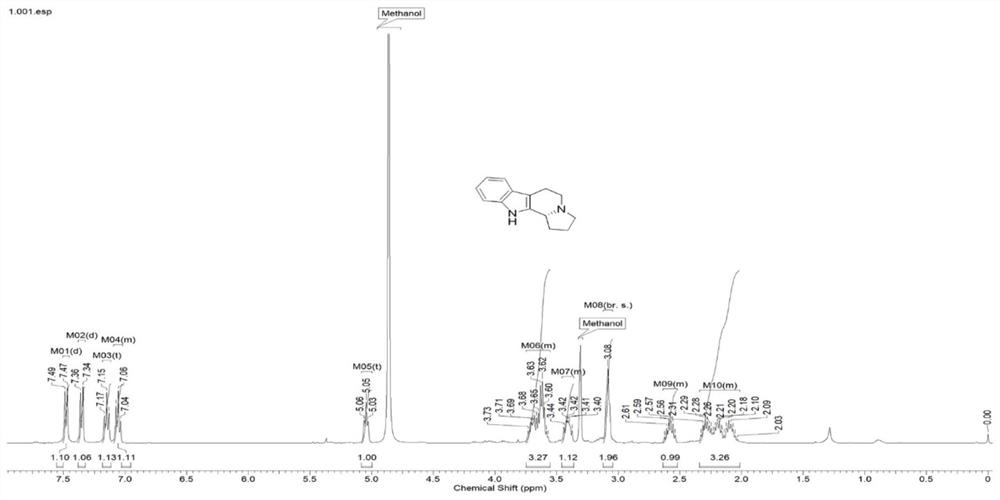
Synthesis method of chiral fused ring tetrahydroisoquinoline alkaloid and analogue thereof
A technology of tetrahydroisoquinoline and dihydroisoquinoline, applied in the direction of microorganism-based methods, biochemical equipment and methods, microorganisms, etc., can solve the problems of selectivity to be improved
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0012] Embodiment 1: Construction and cultivation of genetically engineered bacteria
[0013]The specific construction and cultivation method of the genetically engineered bacteria producing imine reductase is as follows: the amino acid sequence SEQ ID NO: 1 derived from Myxococcusfulvus is gene-synthesized, constructed into a pET series vector, and expressed heterologously in the host bacteria Escherichia coli . Thaw the strains stored at -80°C, streak on the plate, and culture overnight in a constant temperature incubator at 37°C. Select a single colony on the plate and inoculate it into 20mL LB medium containing the corresponding antibiotic, cultivate it for about 12 hours as a seed solution, inoculate it into 700mL LB medium containing the corresponding antibiotic according to the inoculation amount of 1%, and inoculate it on a shaker at 37°C and 200rpm Grow to OD 600 =0.6-0.8, add IPTG with a final concentration of 0.1 mmol / L to induce at 25°C for 12 hours, and collect ...
Embodiment 2
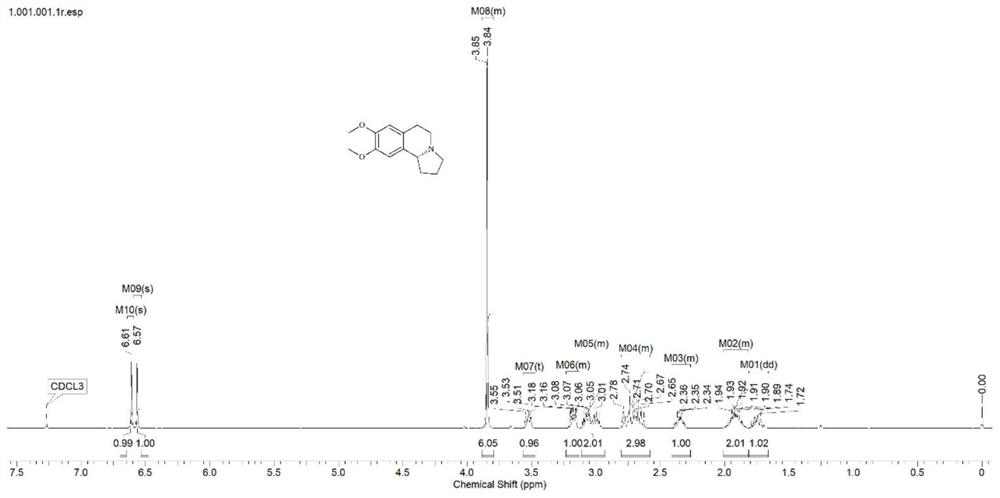
[0014] Embodiment 2: IRED1 catalyzes the preparation of optically pure ( R)-8,9-Dimethoxy-1,2,3,5,6,10b-hexahydropyrrolo[2,1-a]isoquinoline
[0015] 10 mM 8,9-dimethoxy-2,3,5,6-tetrahydro-1H-pyrrolo[2,1-a]isoquinoline-4-ammonium chloride (dissolved in 10% DMSO), 30mM Glucose, 4U / mL GDH, 0.5mg / mL NADP + , 2.50g of IRED1 genetically engineered bacteria, the buffer solution is 100mL of phosphate buffer solution with pH 7.5, reacted at 25°C and 220rpm for 10h. After the reaction, the protein was removed by centrifugation, the supernatant was extracted with ethyl acetate, and the extraction was confirmed by thin-layer chromatography. The organic solvent was removed by rotary evaporation to obtain the crude product. The conversion rate of the substrate and the yield of the product were detected by gas phase, and the ee value of the product detected by liquid phase HPLC was 99%. The conversion rate was 87.5%, the yield was 68.1%, and the configuration of the product was (R).
Embodiment 3
[0016] Example 3: IRED1 catalyzes the preparation of optically pure (R)-9,10-Dimethoxy-2,3,4,6,7,11b-hexahydro-1H-pyrido[2,1-a]isoquinoline
[0017] 30 mM 9,10-dimethoxy-1,2,3,4,6,7-hexahydropyrido[2,1-a]isoquinoline-5-ammonium chloride (dissolved in 10% DMSO) , 90mM Glucose, 12U / mL GDH, 0.5mg / mL NADP + , 2.50g of IRED1 genetically engineered bacteria, the buffer solution is 100mL of phosphate buffer solution with pH 7.5, reacted at 25°C and 220rpm for 10h. After the reaction, the protein was removed by centrifugation, the supernatant was extracted with ethyl acetate, and the extraction was confirmed by thin-layer chromatography. The organic solvent was removed by rotary evaporation to obtain the crude product. The conversion rate of the substrate and the yield of the product were detected by gas phase, and the ee value of the product detected by liquid phase HPLC was 99%. The conversion was 83.3%, the yield was 74.1%, and the configuration of the product was (R).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com