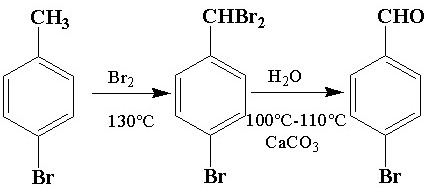
Synthetic method of p-bromobenzaldehyde
A technology of p-bromobenzaldehyde and synthesis method, which is applied in the field of synthesis of p-bromobenzaldehyde, can solve the problems of difficult large-scale production by electro-oxidation method, surrounding environmental pollution, gas and waste liquid discharge, etc., and achieves simple and easy operation, Low cost of raw materials and the effect of reducing emissions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0014] In order to make the above objects, features and advantages of the present invention more comprehensible, the specific implementation of the present invention will be described in detail below in conjunction with specific examples. In the following description, many specific details have been set forth in order to fully understand the present invention, but the present invention can also be implemented in other ways different from those described here, and those skilled in the art can do similar By extension, the present invention is therefore not limited to the specific examples disclosed below.
[0015] The present invention uses p-bromotoluene as raw material, bromination reaction occurs under the catalysis of bromine, wherein the mass ratio of p-bromotoluene to bromine is 1:1-1:2; it is heated to 120-150°C in an oil bath, and after bromine is added , keep the reaction for about 1-3 hours and turn off the heating to obtain 1-bromo-4-(dibromomethyl)benzene, add 200 mL...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com